
Concept explainers
Classify each transformation as substitution, elimination, or addition.
a. 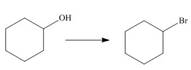 c.
c. 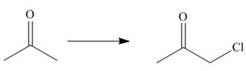
b.  d.
d. 
(a)
Interpretation: The given transformation is to be classified as substitution, elimination, or addition reaction.
Concept introduction: The substitution reactions involve the substitution or replacement of an atom or group of atoms in a compound by another atom or groups of atoms. In the substitution reactions, the replacement takes place by the break down of sigma bonds.
In elimination reaction, the formation of
Answer to Problem 6.1P
The given transformation is an example of a substitution reaction.
Explanation of Solution
The given transformation is shown as,
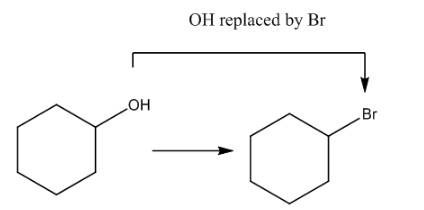
Figure 1
In the above reaction, the hydroxyl group is replaced by a bromine atom. The breakdown of the sigma bond precedes the mechanism. On replacement of the hydroxyl group, one sigma bond breaks and another is formed at the same position. Therefore, it is classified as the substitution reaction.
The given transformation is classified as a substitution reaction.
(b)
Interpretation: The given transformation is to be classified as substitution, elimination, or addition reaction.
Concept introduction: The substitution reactions involve the substitution or replacement of an atom or group of atoms in a compound by another atom or groups of atoms. In the substitution reactions, the replacement takes place by the break down of sigma bonds.
In elimination reaction, the formation of
Answer to Problem 6.1P
The given transformation is an example of an addition reaction.
Explanation of Solution
The given transformation is shown as,
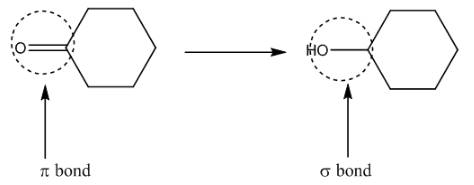
Figure 2
The above reaction involves the breakdown of
The given transformation is classified as an addition reaction.
(c)
Interpretation: The given transformation is to be classified as substitution, elimination, or addition.
Concept introduction: The substitution reactions involve the substitution or replacement of an atom or group of atoms in a compound by another atom or groups of atoms. In the substitution reactions, the replacement takes place by the break down of sigma bonds.
In elimination reaction, the formation of
Answer to Problem 6.1P
The given transformation is an example of a substitution reaction.
Explanation of Solution
The given transformation is shown as,
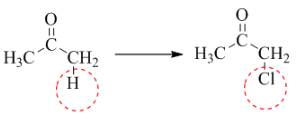
Figure 3
In the above reaction, the hydrogen is replaced by chlorine. The breakdown of the sigma bond precedes the mechanism. On the replacement of hydrogen, one sigma bond breaks and another sigma bond is formed between carbon and substituent atom.
The given transformation is classified as a substitution reaction.
(d)
Interpretation: The given transformation as substitution, elimination, or addition is to be classified.
Concept introduction: The substitution reactions involve the substitution or replacement of an atom or group of atoms in a compound by another atom or groups of atoms. In the substitution reactions, the replacement takes place by the break down of sigma bonds.
In elimination reaction, the formation of
Answer to Problem 6.1P
The given transformation is an example of an elimination reaction.
Explanation of Solution
The given transformation is shown as,
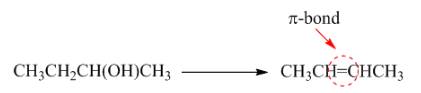
Figure 4
In this reaction, the formation of
The given transformation is classified as an elimination reaction.
Want to see more full solutions like this?
Chapter 6 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Cosmic Perspective Fundamentals
Biology: Life on Earth with Physiology (11th Edition)
SEELEY'S ANATOMY+PHYSIOLOGY
Loose Leaf For Integrated Principles Of Zoology
Organic Chemistry
- Problem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forwardSteps and explanationn please.arrow_forwardSteps and explanationn please.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





