
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6.7P
Use the values in Table 6.2 to calculate
a. 
b. 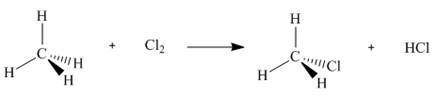
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
0+
aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm
O States of Matter
Sketching a described thermodynamic change on a phase diagram
0/5
Gab
The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the
pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes.
F3
pressure (atm)
0-
0
200
Explanation
temperature (K)
Check
F4
F5
☀+
Q Search
Chill Will an
9
ENG
F6
F7
F8
F9
8
Delete
F10
F11
F12
Insert
PrtSc
114
d
Ar
x +
LEKS: Using a phase diagram a X
n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw
○ States of Matter
Using a phase diagram to find a phase transition temperature or pressure
Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm.
pressure (atm)
16
08-
solid
liquid-
0
200
400
gas
600
temperature (K)
Note: your answer must be within 25 °C of the exact answer to be graded correct.
×
5
S: Using a phase diagram
leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp
O States of Matter
Using a phase diagram to find a phase transition temperature or pressure
se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm.
pressure (atm)
32-
16-
solid
liquid
0.
gas
100
200
temperature (K)
300
Note: your answer must be within 12.5 °C of the exact answer to be graded correct.
10
Explanation
Check
§
Q Search
J
2025 McGraw Hill LLC. All Rights Rese
Chapter 6 Solutions
ORGANIC CHEMISTRY
Ch. 6 - Problem 6.1 Classify each transformation as...Ch. 6 - Prob. 6.2PCh. 6 - Problem 6.3 By taking into account...Ch. 6 - Problem 6.4 Use curved arrows to show the movement...Ch. 6 - Problem 6.5 Follow the curved arrows and draw the...Ch. 6 - Prob. 6.6PCh. 6 - Problem 6.7 Use the values in Table 6.2 to...Ch. 6 - Prob. 6.8PCh. 6 - aWhich Keq corresponds to a negative value of G,...Ch. 6 - Given each of the following values, is the...
Ch. 6 - Given each of the following values, is the...Ch. 6 - The equilibrium constant for the conversion of the...Ch. 6 - Prob. 6.13PCh. 6 - For a reaction with H=40kJ/mol, decide which of...Ch. 6 - For a reaction with H=20kJ/mol, decide which of...Ch. 6 - Draw an energy diagram for a reaction in which the...Ch. 6 - Prob. 6.17PCh. 6 - Prob. 6.18PCh. 6 - Problem 6.19 Consider the following energy...Ch. 6 - Draw an energy diagram for a two-step reaction,...Ch. 6 - Which value if any corresponds to a faster...Ch. 6 - Prob. 6.22PCh. 6 - Problem 6.23 For each rate equation, what effect...Ch. 6 - Prob. 6.24PCh. 6 - Identify the catalyst in each equation. a....Ch. 6 - Draw the products of homolysis or heterolysis of...Ch. 6 - Explain why the bond dissociation energy for bond...Ch. 6 - Classify each transformation as substitution,...Ch. 6 - Prob. 6.29PCh. 6 - 6.30 Draw the products of each reaction by...Ch. 6 - 6.31 (a) Add curved arrows for each step to show...Ch. 6 - Prob. 6.32PCh. 6 - Prob. 6.33PCh. 6 - Prob. 6.34PCh. 6 - Calculate H for each reaction. a HO+CH4CH3+H2O b...Ch. 6 - Homolysis of the indicated CH bond in propene...Ch. 6 - Prob. 6.37PCh. 6 - Prob. 6.38PCh. 6 - 6.39. a. Which value corresponds to a negative...Ch. 6 - Prob. 6.40PCh. 6 - For which of the following reaction is S a...Ch. 6 - Prob. 6.42PCh. 6 - Prob. 6.43PCh. 6 - 6.44 Consider the following reaction: .
Use curved...Ch. 6 - Prob. 6.45PCh. 6 - Draw an energy diagram for the Bronsted-Lowry...Ch. 6 - Prob. 6.47PCh. 6 - Indicate which factors affect the rate of a...Ch. 6 - Prob. 6.49PCh. 6 - 6.50 The conversion of acetyl chloride to methyl...Ch. 6 - Prob. 6.51PCh. 6 - Prob. 6.52PCh. 6 - The conversion of (CH3)3Cl to (CH3)2C=CH2 can...Ch. 6 - 6.54 Explain why is more acidic than , even...Ch. 6 - Prob. 6.55PCh. 6 - Prob. 6.56PCh. 6 - Prob. 6.57PCh. 6 - Although Keq of equation 1 in problem 6.57 does...Ch. 6 - Prob. 6.59P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Identify me theme or themes exemplified by (a) the sharp quills of a porcupine (b) the development of a multice...
Campbell Biology in Focus (2nd Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardResults Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
- Search Results Search Results Best Free Coursehero Unlo x b Success Confirmation of Q aleks.com/alekscgi/x/sl.exe/10_u-lgNslkr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTIOHz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCav States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 0. 32- 16 solid liquid gas 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Дос Xarrow_forwardConsider the reaction below to answer the following questions: Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. 1. NaOEt, EtOH H&C OCH CH3 2 H30 H3C CH2 OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. OEtarrow_forwardUse the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 3.7 atm. pressure (atm) 32 16 solid liquid gas 0 0 200 temperature (K) Note: your answer must be within 20 °C of the exact answer to be graded correct. Шос ☑ كarrow_forward
- Starting from bromoethane, how could you prepare the following compounds: a. Ethanol. b. Acetaldehyde f. Acetone. e. 2-Propanol i. Acetoacetic ester. d. 2-Bromoacetic acid. c. Acetic acid g. Acetamide. j. Ethylmalonate k. Gama ketoacid. h. Ethyl magnesium bromide.arrow_forward- The pressure above a pure sample of solid Substance X at 60. °C is raised. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 02 0.4 solid Hliquid gas 0 0 200 400 600 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. ☐ atmarrow_forward15. What is the order of decreasing reactivity towards nucleophilic acyl substitution for the carboxylic acid derivatives? (most reactive first) 0 O H3C COC CH3 H₂C C N(CH3)2 H3C C OCH3 A. a. I, 11, 111, b. I, III, IV, II C. II, IV, III, I ° (CH3)2CH C OCH3 IV d. II, I, III, IV B. R COCR 0 0 0 13= RC NH2 RC OR RC CI === IV a. I, III, II, IV b. II, III, I, IV C. III, II, I, IV d. IV, I, III, IIarrow_forward
- Draw the formula of the product obtained by reacting D-Tallose with bromine water.arrow_forwardChoose the best reagent(s) for carrying out the following conversions from the list below. Place the letter corresponding to the best choice in the blank to the left of the conversion. a. KMnO4, H3O+ b. Tollens' Reagent [oxidizing reagent] C. NaBH4, ethanol d. 1. BH3 2. H3O+ e. 1. CH3MgBr, ether 2. H3O+ f. CrO3, H2SO4, H₂O g. 1. Mg, ether 2. CO2 3. H3O+ h. 1. NaCN 2. H2SO4, H2, heat i. O3, then Zn and HOAC j. CH₂I A. B. C. CH CH=CHCH2COOH Br CEN CH COOH + HOOCCH COOH COOH 010 CH3arrow_forwardDraw the structures for each of the intermediates in the boxes provided for the synthesis below. OCH3 Fe HO HNO (CHOO pynding H₂504 LHNO2 NACH-I Fa H₂O HCL HNO 180arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY