
(a)
Interpretation:
The product formed when cis- and
Concept introduction:
Addition of hydrogen halides:
In the addition of hydrogen halides over
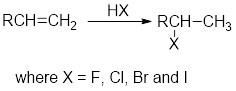
Carbocation: carbon ion that bears a positive charge on it.
Carbocation stability order:

Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Chiral or Asymmetric carbon: Carbon bonded with four different substituents with it is termed as chiral or asymmetric carbon.
(b)
Interpretation:
The product formed when cis- and
Concept introduction:
Addition of
Catalytic hydrogenation in presence of
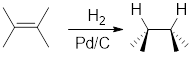
For cyclic reactants, the addition of
(c)
Interpretation:
The product formed when cis- and
Concept introduction:
Acid Catalyzed addition of water: When water is added to alkene in the presence of an acid, the product formed will be an alcohol.
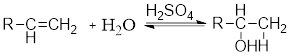
The electrophile
Nucleophile: It donates pair of electrons to positively charged chemical substrate resulting in the formation of
Electrophile: It is positively charged species which seeks for negative charge and hence accepts pair of electrons from negatively charged species (Nucleophiles) which results in the formation of chemical bond.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


