
(a)
Interpretation:
The electron sublevel starts to fill after the completion of 5s sublevel should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
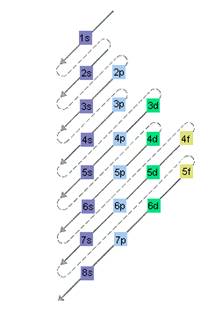
(b)
Interpretation:
The electron sublevel starts to fill after the completion of 4d sublevel should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
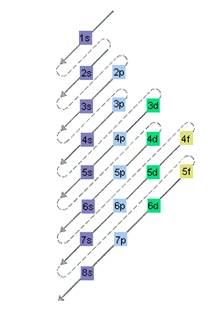
(c)
Interpretation:
The electron sublevel starts to fill after the completion of 4f sublevel should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
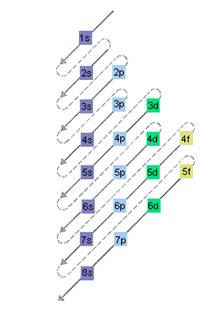
(d)
Interpretation:
The electron sublevel starts to fill after the completion of 5p sublevel should be determined.
Concept Introduction:
The rules for the allowed quantum numbers combinations are as follows:
- All the three quantum numbers ( n, l and m ) describes the orbital of an atom and they are integers.
- The principal quantum number, n value cannot be zero. Thus, the values allowed for the principal quantum number are 1, 2, 3, 4, and so on.
- The value of angular quantum number, l can be between 0 to n-1. Thus, if value of n is equal to 3 the value of l can be 0, 1 or 2.
- The value of magnetic quantum number, m can be between − l to +l . Thus, if value of l is equal to 2, m can be wither -2, -1, 0, +1, or +2
Here, for s orbital value of
The relative energy of orbitals is represented as follows:
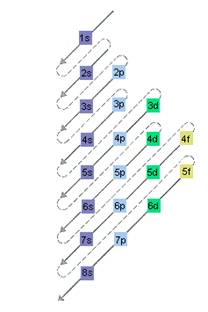
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Basic Chemistry (5th Edition)
- Label each of the seven designated regions of the following multi-component, solid-liquid phase diagram for the Zinc - Magnesium system.arrow_forward22arrow_forwardPLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS FOR THE MECHANISM!!! THANKS First image: QUESTION 6. I have to show, with ARROWS and STRUCTURES, the mechanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary. I also tried to draw the mechanism, tell me what to change. Please note that its an AMIDE thats formed not an AMINE the nitrogen has ONE hydrogen and one Phenyl-C-Phenyl. I already asked for this mechanism and got as a final product ...-NH2 not whats shown on the picture, thank you Ths second part. QUESTION 3. I just need a way to synthesize the lactone A, I already started please continue from where I left it Second image: I simply need the products, substrates or reagents, thank youarrow_forward
- Indicate how to prepare a 10% sodium hydroxide (NaOH) solution to a slightly alkaline pH.arrow_forwardCH, CH CH₂ CH₂ Phytyl side chain 5. What is the expected order of elution of compounds A-D below from a chromatography column packed with silica gel, eluting with hexane/ethyl acetate? C D OHarrow_forwardPlease analze my gel electrophoresis column of the VRK1 kinase (MW: 39.71 kDa). Attached is the following image for the order of column wells and my gel.arrow_forward
- 2.0arrow_forwardWrite the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 5 6 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! ☐arrow_forwardCompare these chromatograms of three anti-psychotic drugs done by HPLC and SFC. Why is there the difference in separation time for SFC versus HPLC? Hint, use the Van Deemter plot as a guide in answering this question. Why, fundamentally, would you expect a faster separation for SFC than HPLC, in general?arrow_forward
- A certain inorganic cation has an electrophoretic mobility of 5.27 x 10-4 cm2s-1V-1. The same ion has a diffusion coefficient of 9.5 x 10-6cm2s-1. If this ion is separated from cations by CZE with a 75cm capillary, what is the expected plate count, N, at an applied voltage of 15.0kV? Under these separation conditions, the electroosmotic flow rate was 0.85mm s-1 toward the cathode. If the detector was 50.0cm from the injection end of the capillary, how long would it take in minutes for the analyte cation to reach the detector after the field was applied?arrow_forward2.arrow_forwardPlease solve for the following Electrochemistry that occursarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





