
Concept explainers
(a)
Interpretation:
The elements with the given electronic configuration should be identified as a metal, non-metal or metalloid.
Concept Introduction:
The elements which can donate electrons to get the stable electronic configuration are metal, elements that can accept electrons are non-metal. The elements with characteristics of both metals and non-metals are metalloids.
(b)
Interpretation:
The element with the smallest atomic size should be identified.
Concept Introduction:
According to the periodic trends, the atomic size of the element increases on moving top to bottom in a group due to addition of one shell and decreases on moving left to right in a group due to increase in
(c)
Interpretation:
The element with the highest ionization energy should be identified.
Concept Introduction:
Ionization energy is defined as the amount of energy required to remove an electron from its outermost shell. According to periodic trends, it decreases on moving top to bottom in a group due to increase in size and it increases on moving left to right in a period due to decrease in size.
(d)
Interpretation:
The element with the half-filled sublevel should be identified.
Concept Introduction:
The relative energy of orbitals is represented as follows:
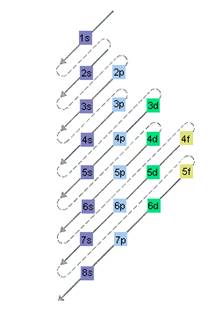
A s orbital can have maximum of 2 electrons, p orbital can have maximum of 6 electrons. Similarly, maximum electrons that a d and f orbital can have are 10 and 14 respectively.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Basic Chemistry (5th Edition)
- 5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forward
- Draw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forward
- Explain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forwardExplain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forward
- Briefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forwardGiven the hydrazones indicated, draw the structures of the enamines that can be formed. Indicate the most stable enamine (explain). C6H5 C6H5 H C6H5 Harrow_forward4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





