
Experimental Organic Chemistry: A Miniscale & Microscale Approach (Cengage Learning Laboratory Series for Organic Chemistry)
6th Edition
ISBN: 9781305080461
Author: John C. Gilbert, Stephen F. Martin
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Question
Chapter 4.6, Problem 7E
Interpretation Introduction
Interpretation:Number of five-carbon units with below skeletal structure in geranial and vitamin A should be determined.
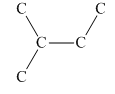
Concept introduction:Terpenes are varieties of organic compounds that are synthesized by plants. These are hydrocarbons that can be found in several plants and trees. These act as constituents for different oils.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The conversion of (CH3)3CI to (CH3)2C=CH2 can occur by either a one-step or a two-step mechanism, as shown in
Equations [1] and [2].
[1]
+ I +
H₂Ö:
:OH
[2]
q
slow
:OH
+ I¯
H₂Ö:
a. What rate equation would be observed for the mechanism in Equation [1]?
b. What rate equation would be observed for the mechanism in Equation [2]?
c. What is the order of each rate equation (i.e., first, second, and so forth)?
d. How can these rate equations be used to show which mechanism is the right one for this reaction?
e. Assume Equation [1] represents an endothermic reaction and draw an energy diagram for the reaction. Label the
axes, reactants, products, Ea, and AH°. Draw the structure for the transition state.
f. Assume Equation [2] represents an endothermic reaction and that the product of the rate-determining step is higher
in energy than the reactants or products. Draw an energy diagram for this two-step reaction. Label the axes,
reactants and products for each step, and the Ea and AH° for each…
Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.
Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.
Chapter 4 Solutions
Experimental Organic Chemistry: A Miniscale & Microscale Approach (Cengage Learning Laboratory Series for Organic Chemistry)
Ch. 4.2 - Prob. 1ECh. 4.2 - Prob. 2ECh. 4.2 - Prob. 3ECh. 4.2 - Prob. 4ECh. 4.2 - Prob. 5ECh. 4.2 - Prob. 6ECh. 4.2 - Prob. 7ECh. 4.2 - Prob. 8ECh. 4.2 - Prob. 9ECh. 4.3 - Prob. 1E
Ch. 4.3 - Prob. 2ECh. 4.3 - Prob. 3ECh. 4.3 - Prob. 4ECh. 4.3 - Prob. 5ECh. 4.3 - Prob. 6ECh. 4.3 - Prob. 7ECh. 4.3 - Prob. 8ECh. 4.4 - Prob. 1ECh. 4.4 - Prob. 2ECh. 4.4 - Prob. 3ECh. 4.4 - Prob. 4ECh. 4.4 - Prob. 5ECh. 4.4 - Prob. 6ECh. 4.4 - Prob. 7ECh. 4.4 - Prob. 8ECh. 4.4 - Prob. 9ECh. 4.4 - Prob. 11ECh. 4.4 - Prob. 12ECh. 4.4 - Prob. 13ECh. 4.4 - Prob. 14ECh. 4.6 - Prob. 1ECh. 4.6 - Prob. 2ECh. 4.6 - Prob. 3ECh. 4.6 - Prob. 4ECh. 4.6 - Prob. 5ECh. 4.6 - Prob. 6ECh. 4.6 - Prob. 7ECh. 4.6 - Prob. 8ECh. 4.6 - Prob. 9E
Knowledge Booster
Similar questions
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardFor a complex reaction with the rate equation v = k1[A] + k2[A]2, we can say(A) that it is of order 1.(B) that it is of order 1.5.(C) that it is of order 2.(D) that for certain values of [A] it can behave as if it were of order 1, and for other values as if it were of order 2.arrow_forwarda. Draw a complete arrow pushing mechanism for the following. Is this the thermodynamic or the kinetic product? Use your mechanism to explain your choice. Draw all the resonance. HBr Brarrow_forward
- Which, if any, of the substances had resonance structures? How many resonance structures did each substance have from the following list: CCl4 H2O CO2 C2H4 NH3 SF6 ICl5arrow_forwardSteps and explanation pleasearrow_forwardSteps and explanation please. Add how to solve or target similar problems.arrow_forward
- Steps and explanation please. Add how to solve or target similar problems.arrow_forwardSteps and explanation please. Add how to solve or target similar problems.arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning