
Experimental Organic Chemistry: A Miniscale & Microscale Approach (Cengage Learning Laboratory Series for Organic Chemistry)
6th Edition
ISBN: 9781305080461
Author: John C. Gilbert, Stephen F. Martin
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4.6, Problem 3E
Interpretation Introduction
Interpretation:Structures for semicarbazone and 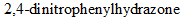 of citral should be determined.
of citral should be determined.
Concept introduction:Citral is natural oil that is composed of two isomeric forms, geranial and neral. It has odor and taste similar to that of lemon. It acts as defense pheromone, used in several perfumes and also plays crucial role in synthesis of vitamin A.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the products of this organic reaction:
CH3-C-O-CH2-CH2-C-CH3
+ H₂O
?
A
Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them
in any arrangement you like, so long as they aren't touching.)
If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area.
No reaction
Click anywhere to draw the first
atom of your structure.
:☐
d
DE
d. Draw an arrow pushing mechanism for the following
IN
O
CI
N
fo
人
P
Polle
DELL
prt sc
home
end
ins
F5
F6
F7
F8
F9
F10
F11
F12
Predict the products of this organic reaction:
+ H₂O H* ?
A
Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw
them in any arrangement you like, so long as they aren't touching.)
If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area.
No Reaction
Click and drag to start drawing a
structure.
Chapter 4 Solutions
Experimental Organic Chemistry: A Miniscale & Microscale Approach (Cengage Learning Laboratory Series for Organic Chemistry)
Ch. 4.2 - Prob. 1ECh. 4.2 - Prob. 2ECh. 4.2 - Prob. 3ECh. 4.2 - Prob. 4ECh. 4.2 - Prob. 5ECh. 4.2 - Prob. 6ECh. 4.2 - Prob. 7ECh. 4.2 - Prob. 8ECh. 4.2 - Prob. 9ECh. 4.3 - Prob. 1E
Ch. 4.3 - Prob. 2ECh. 4.3 - Prob. 3ECh. 4.3 - Prob. 4ECh. 4.3 - Prob. 5ECh. 4.3 - Prob. 6ECh. 4.3 - Prob. 7ECh. 4.3 - Prob. 8ECh. 4.4 - Prob. 1ECh. 4.4 - Prob. 2ECh. 4.4 - Prob. 3ECh. 4.4 - Prob. 4ECh. 4.4 - Prob. 5ECh. 4.4 - Prob. 6ECh. 4.4 - Prob. 7ECh. 4.4 - Prob. 8ECh. 4.4 - Prob. 9ECh. 4.4 - Prob. 11ECh. 4.4 - Prob. 12ECh. 4.4 - Prob. 13ECh. 4.4 - Prob. 14ECh. 4.6 - Prob. 1ECh. 4.6 - Prob. 2ECh. 4.6 - Prob. 3ECh. 4.6 - Prob. 4ECh. 4.6 - Prob. 5ECh. 4.6 - Prob. 6ECh. 4.6 - Prob. 7ECh. 4.6 - Prob. 8ECh. 4.6 - Prob. 9E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forwardYou are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forward
- Predict the products of this organic reaction: CH3 O CH3-CH-C-O-CH2-CH2-CH3 + H₂OH+ Η ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward€ CH3-CH-C-O-CH2-CH2-CH3 + NaOH A? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Predict the products of this organic reaction: CH3 O Click anywhere to draw the first atom of your structure. No reaction ✓ Garrow_forwardA molecule can have a temporary or permanent depending on the structure and the way the electrons can move. True Falsearrow_forward
- edict the products of this organic reaction: CH3 O A CH3-CH-C-NH2 + H2O + HCI ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Click anywhere to draw the first atom of your structure. No Reaction planation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center +arrow_forwardDraw the mechanism for the following reaction: OH A few notes: CI O • You may assume that each reagent is present in whatever amount you need to draw your mechanism. • To save you some time, one of the starting materials has been copied into the first step of the drawing area. AP Add/Remove step Cl Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? CH3 O [0] R CH3-CH-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note: the organic equation above only shows the important organic reactant and product. Minor small-molecule reactants or products (like H₂O) are not shown. No Answer Click anywhere to draw the first atom of your structure. ×arrow_forward
- Predict the product of this organic reaction: O CH3 A NH3 + HO–C—CH—CH, P+H₂O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. A 5arrow_forwardFor a reaction to be spontaneous, the Gibbs Free Energy must be less than zero. True or Falsearrow_forwardWhich of the following reactions will be exothermic? a) Reaction has enthalpy less than zero. b) Reaction has enthalpy greater than zero. c) Reaction has entropy less than zero. d) Reaction has entropy equal to zero.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY