
Concept explainers
(a)
Energy level diagram for one-dimensional box.
(a)
Answer to Problem 7P
The energy level diagram is given below:
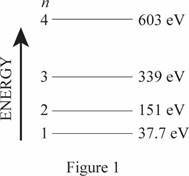
Explanation of Solution
The length of the box is
Write the expression for energy of one dimensional box
Here,
Conclusion
Substitute
Substitute
Substitute
Substitute
The energy level diagram is given below:
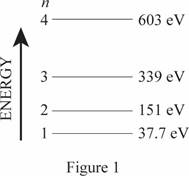
(b)
The wavelength of emitted photons during transitions in these four levels.
(b)
Answer to Problem 7P
The frequencies of the emitted photon are
Explanation of Solution
The minimum frequency for photoemission corresponds to the cutoff wavelength.
Write the expression for wavelength
Here,
Conclusion
For transition,
Substitute
For transition,
Substitute
For transition,
Substitute
For transition,
Substitute
For transition,
Substitute
For transition,
Substitute
Thus, the frequencies of the emitted photon are
Want to see more full solutions like this?
Chapter 41 Solutions
Physics for Scientists and Engineers with Modern Physics, Technology Update
- Need help wity equilibrium qestionarrow_forwardneed answer asap please thanks youarrow_forwardA man slides two boxes up a slope. The two boxes A and B have a mass of 75 kg and 50 kg, respectively. (a) Draw the free body diagram (FBD) of the two crates. (b) Determine the tension in the cable that the man must exert to cause imminent movement from rest of the two boxes. Static friction coefficient USA = 0.25 HSB = 0.35 Kinetic friction coefficient HkA = 0.20 HkB = 0.25 M₁ = 75 kg MB = 50 kg P 35° Figure 3 B 200arrow_forward
- A golf ball is struck with a velocity of 20 m/s at point A as shown below (Figure 4). (a) Determine the distance "d" and the time of flight from A to B; (b) Determine the magnitude and the direction of the speed at which the ball strikes the ground at B. 10° V₁ = 20m/s 35º Figure 4 d Barrow_forwardThe rectangular loop of wire shown in the figure (Figure 1) has a mass of 0.18 g per centimeter of length and is pivoted about side ab on a frictionless axis. The current in the wire is 8.5 A in the direction shown. Find the magnitude of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane. Find the direction of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane.arrow_forwardA particle with a charge of − 5.20 nC is moving in a uniform magnetic field of (B→=−( 1.22 T )k^. The magnetic force on the particle is measured to be (F→=−( 3.50×10−7 N )i^+( 7.60×10−7 N )j^. Calculate the y and z component of the velocity of the particle.arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College





