
Concept explainers
Calculate the gas pressure inside the platinum capsule after
Answer to Problem 4.35P
Gas pressure is
Explanation of Solution
When Radium-226 decay, it emits a particles each a particle is a helium nucleus with 2 electrons and forms helium gas which produces pressure in a capsule.
226 Ra emits 4 alpha particles per decay immediately after secular equilibrium and there is the formation of the fifth a particle.
The fraction of
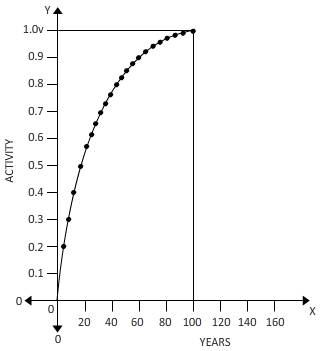
Above carve shows a fraction of a to helium over 100 years.
Fraction=Area under curve/Total area
=
Integrating from
Fraction=
Four alpha particles emitted over
We know that,
To calculate the fraction of decay
Rearranging, (1) and (2)
Hence
To calculate helium atoms formed over
We have,
The dimension of the capsule,
The molecular weight of RaBr2,
The volume of RaBr2 is,
Volume available for gas=volume of capsule-volume of RaBr2
According to Ideal gas low
The pressure of helium formed by the decay of radium is 22.3 atmospheres. But originally capsule contains an air of 1 atmospheric pressure. Hence this initial pressure must be considered.
Thus, pressure=
Conclusion:
We can conclude about this problem that, when Radium-226 decays. It emits a particles having helium nucleus with two electrons and produces helium gas. Ideal gas law is used to calculate the pressure of helium gas. The gas pressure inside the capsule is 23.3 atm due to the formation of helium gas by the decay of radium.
Want to see more full solutions like this?
Chapter 4 Solutions
Introduction To Health Physics
- How can I remember this Formula: p = m × v where m is in kg and v in Meter per second in the best way?arrow_forwardHow can I remember the Formula for the impulsearrow_forwardA Geiger-Mueller tube is a radiation detector that consists of a closed, hollow, metal cylinder (the cathode) of inner radius ra and a coaxial cylindrical wire (the anode) of radius г (see figure below) with a gas filling the space between the electrodes. Assume that the internal diameter of a Geiger-Mueller tube is 3.00 cm and that the wire along the axis has a diameter of 0.190 mm. The dielectric strength of the gas between the central wire and the cylinder is 1.15 × 106 V/m. Use the equation 2πrlE = 9in to calculate the maximum potential difference that can be applied between the wire and the cylinder before breakdown occurs in the gas. V Anode Cathodearrow_forward
- 3.77 is not the correct answer!arrow_forwardA I squar frame has sides that measure 2.45m when it is at rest. What is the area of the frame when it moves parellel to one of its diagonal with a m² speed of 0.86.c as indicated in the figure? >V.arrow_forwardAn astronent travels to a distant star with a speed of 0.44C relative to Earth. From the austronaut's point of view, the star is 420 ly from Earth. On the return trip, the astronent travels speed of 0.76c relative to Earth. What is the distance covered on the return trip, as measured by the astronant? your answer in light-years. with a Give ly.arrow_forward
- star by spaceship Sixus is about 9.00 ly from Earth. To preach the star in 15.04 (ship time), how fast must you travel? C.arrow_forwardIf light-bulb A is unscrewed, how will the brightness of bulbs B and C change, if at all? How does the current drawn by from the battery change?arrow_forwardCan someone help mearrow_forward
- Can someone help me with this thank youarrow_forward(a) For a spherical capacitor with inner radius a and outer radius b, we have the following for the capacitance. ab C = k₂(b- a) 0.0695 m 0.145 m (8.99 × 10º N · m²/c²)( [0.145 m- 0.0695 m × 10-11 F = PF IIarrow_forwardA pendulum bob A (0.5 kg) is given an initialspeed of vA = 4 m/s when the chord ishorizontal. It then hits a stationary block B (1kg) which then slides to a maximum distanced before it stops. Determine the value of d.The coefficient of static friction between theblock and the plane is μk = 0.2. The coefficientof restitution between A and B is e = 0.8.Ans: d=1.0034 marrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning





