
Concept explainers
3-78 Nitrous oxide, N20, laughing gas, is a colorless, nontoxic, tasteless, and odorless gas. It is used as an inhalation anesthetic in dental and other surgeries. Because nitrous oxide is soluble in vegetable oils (fats), it is used commercially as a propellant in whipped toppings
Nitrous oxide dissolves in fats. The gas is added under pressure to cans of whipped topping. When the valve is opened, the gas expands, thus expanding (whipping) the topping and forcing it out of the can.
(a) How many valence electrons are present in a molecule of N20?
(b) Write two equivalent contributing structures for this molecule. The connectivity in nitrous oxide is N−N−O.
(c) Explain why the following is not an acceptable contributing structure:
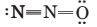
Trending nowThis is a popular solution!

Chapter 3 Solutions
Introduction To General, Organic, And Biochemistry
- 3-119 Perchloroethylene, which is a liquid at room temperature, is one of the most widely used solvents for commercial dry cleaning. It is sold for this purpose under several trade names, including Perciene®. Does this molecule have polar bonds? Is it a polar molecule? Does it have a dipole?arrow_forward3-63 What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion? Draw the Lewis structure for each.arrow_forward3-48 Potassium chloride and potassium bicarbonate are used as potassium dietary supplements. Write the formula of each compound.arrow_forward
- Write the empirical formula for at least four ionic compounds that could be formed from the following ions: 3+ NH,, PO, Fe”, CH, CO, 0 0,0,... X Śarrow_forward(a) Flake white was a white pigment used by Renaissance artists, which was composed of a combination of lead(II) carbonate and lead(II) hydroxide. What are the correct chemical formulas for these two compounds? PbHCO3 Pb(OH)2 Pb2CO3 PbO Pb(OH)4 PbCO3arrow_forwardWrite the empirical formula for at least four ionic compounds that could be formed from the following ions: 2+ 4+ OH, Fe, CN , Pb' 0,0,...arrow_forward
- Some salts make hydrates when there is moisture around. Some hydrate samples can absorb lights in visible legion. Therefore, they show some colors. Thus, hydrates can be used to detect the moisture in the environment. Let's say you are working in a laboratory with a group and you are the only student who has taken chemistry courses. Your laboratory received a sample of cobalt(II) chloride which has the formula CoCl2·xH2O. Let's say your boss asked you to find the formula of this hydrate salt sample since you are the only chemist there. From your laboratory experience, simply explain the experimental procedure you would follow to find the formula of this unknown sample. Then, use the given data for the calculation part. Mass of crucible: 32.27g Mass of crucible + unknown hydrate: 33.92 g Color of unknown hydrate: purple Mass of crucible + anhydrous form of hydrate: 33.41 g Color of anhydrous form: sky bluearrow_forwardWrite the empirical formula of at least four binary ionic compounds that could be formed from the following ions: Zn²*, Ar**, Br¯ , s²- 3+ , Al*, Br, s-arrow_forwardAlthough not a transition metal, lead can form Pb21 and Pb41 ions. Write the formula for the compound formed between each of these lead ions and the following anion: (a) Oxide ionarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





