
Concept explainers
3-77 Ozone, O3, is an unstable blue gas with a characteristic pungent odor. In an ozone molecule, the connectivity of the atoms is O−O−O and both O−O bonds are equivalent.
(a) How many valence electrons must be present in an acceptable Lewis structure for an ozone molecule?
(b) Write two equivalent resonance contributing structures for ozone. Be certain to show any positive or negative charges that may be present in your contributing structures. By equivalent contributing structures, we mean that each has the same pattern of bonding.
(c) Show by the use of curved arrows how the first of your contributing structures may be converted to the second.
(d) Based on your contributing structures, predict the O−O−O bond angle in ozone.
(e) Explain why the following is not an acceptable contributing structure for an ozone molecule:
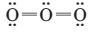
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Introduction to General, Organic and Biochemistry
- Please correct answer and don't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardDetermine whether the following reaction is an example of a nucleophilic substitution reaction: Br OH HO 2 -- Molecule A Molecule B + Br 义 ollo 18 Is this a nucleophilic substitution reaction? If this is a nucleophilic substitution reaction, answer the remaining questions in this table. Which of the reactants is referred to as the nucleophile in this reaction? Which of the reactants is referred to as the organic substrate in this reaction? Use a ŏ + symbol to label the electrophilic carbon that is attacked during the substitution. Highlight the leaving group on the appropriate reactant. ◇ Yes O No O Molecule A Molecule B Molecule A Molecule B टेarrow_forward
- Show work..don't give Ai generated solutionarrow_forwardShow work..don't give Ai generated solutionarrow_forwardPheromone G of the maize stalk borer, chilo partelus, can be synthesized based on the partial scheme shown below. Complete the scheme by identifying the structures of the intermediate compounds A, B, C, D, E, F and pheromone G. Indicate stereochemistry where relevantarrow_forward
- Q8: Draw the resonance structures for the following molecule. Show the curved arrows (how you derive each resonance structure). Circle the major resonance contributor. одarrow_forwardQ9: Explain why compound I is protonated on O while compound II is protonated on N. NH2 DD I II NH2arrow_forwardComplete the following reaction by identifying the principle organic product of the reactionarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
