
Concept explainers
(a)
Interpretation:
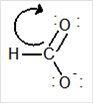
Draw the contributing structure of.
Concept Introduction:
Lewis electron dot symbol is a structure of a molecule that shows the bonding between atoms as well as lone pairs of electrons of atoms also.
Valence electron: The electrons which are present in the outer most energy level are known as valence electron. This can be calculated by the group number of the element. Generally the group number is same as the valence electrons of any elements.
(b)
Interpretation:
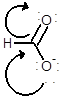
Draw the contributing structure of.
Concept Introduction:
Lewis electron dot symbol is a structure of a molecule that shows the bonding between atoms as well as lone pairs of electrons of atoms also.
Valence electron: The electrons which are present in the outer most energy level are known as valence electron. This can be calculated by the group number of the element. Generally the group number is same as the valence electrons of any elements.
(c)
Interpretation:
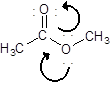
Draw the contributing structure of.
Concept Introduction:
Lewis electron dot symbol is a structure of a molecule that shows the bonding between atoms as well as lone pairs of electrons of atoms also.
Valence electron: The electrons which are present in the outer most energy level are known as valence electron. This can be calculated by the group number of the element. Generally the group number is same as the valence electrons of any elements.
Trending nowThis is a popular solution!

Chapter 3 Solutions
Introduction to General, Organic and Biochemistry
- =Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forwardI dont understand this.arrow_forward
- Can you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!arrow_forwardBasic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forward
- The answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forwardDraw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

