
Introduction to General, Organic and Biochemistry
12th Edition
ISBN: 9780357391594
Author: Frederick A. Bettelheim; William H. Brown; Mary K. Campbell
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 89P
3-105 Consider the structure of Vitamin E shown below, which is found most abundantly in wheat germ oil, sunflower, and safflower oils:
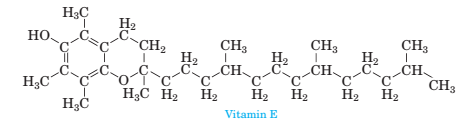
(a) Identify the various types of geometries present in each central atom using VSEPR theory.
(b) Determine the various relative bond angles as sociated with each central atom using VSEPR theory.
(c) Which is the most polar bond in Vitamin E?
(d) Would you predict Vitamin E to be polar or nonpolar?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please help me answer number 1. 1. If your graphs revealed a mathematical relationship between specific heat and atomic mass, write down an equation for the relationship.
I also don't understand, is the equation from the line regression the one that I'm suppose use to show the relationship? If so could you work it all the way out?
Describe the principle of resonance and give a set of Lewis Structures to illustrate your explanation.
Don't used hand raiting
Chapter 3 Solutions
Introduction to General, Organic and Biochemistry
Ch. 3.1 - Problem 3-1 Show how the following chemical...Ch. 3.3 - Problem 3-2 Judging from their relative positions...Ch. 3.4 - Problem 3-3 Write the formulas for the ionic...Ch. 3.5 - Problem 3-4 Name these binary ionic compounds: (a)...Ch. 3.5 - Prob. 3.5QCCh. 3.5 - Problem 3-6 Give each binary compound a systematic...Ch. 3.5 - Problem 3-7 Name these ionic compounds, each of...Ch. 3.6 - Prob. 3.8QCCh. 3.6 - Prob. 3.9QCCh. 3.6 - Prob. 3.10QC
Ch. 3.6 - Prob. 3.11QCCh. 3.7 - Prob. 3.12QCCh. 3.8 - Prob. 3.13QCCh. 3.8 - Prob. 3.14QCCh. 3.9 - Problem 3-15 Predict all bond angles for these...Ch. 3.10 - Problem 3-16 Which of these molecules are polar?...Ch. 3 - 3-17 Answer true or false. (a) The octet rule...Ch. 3 - 3-18 How many electrons must each atom gain or...Ch. 3 - 3-19 Show how each chemical change obeys the octet...Ch. 3 - 3-20 Show how each chemical change obeys the octet...Ch. 3 - 3-21 Write the formula for the most stable ion...Ch. 3 - 3-22 Why is Li- not a stable ion?Ch. 3 - 3-23 Predict which ions are stable: (a) (b) (c)...Ch. 3 - 3-24 Predict which ions are stable: (a) Br2- (b)...Ch. 3 - 3-25 Why are carbon and silicon reluctant to form...Ch. 3 - 3-26 Table 3-2 shows the following ions of copper:...Ch. 3 - 3-27 Answer true or false. (a) For Group lA and...Ch. 3 - 3-28 Name each polyatomic ion. (a) HCO3- (b) NO2-...Ch. 3 - 3-29 Answer true or false. (a) According to the...Ch. 3 - Prob. 14PCh. 3 - 3-31 Why does electronegativity generally increase...Ch. 3 - 3-32 Judging from their relative positions in the...Ch. 3 - Prob. 17PCh. 3 - 3-34 Which of these bonds is the most polar? The...Ch. 3 - 3-35 Classify each bond as nonpolar covalent,...Ch. 3 - 3-36 Classify each bond as nonpolar covalent,...Ch. 3 - 3-37 Answer true or false. (a) An ionic bond is...Ch. 3 - 3-38 Complete the chart by writing formulas for...Ch. 3 - 3-39 Write a formula for the ionic compound formed...Ch. 3 - Prob. 24PCh. 3 - 3-41 Describe the structure of sodium chloride in...Ch. 3 - 3-42 What is the charge on each ion in these...Ch. 3 - 3-43 Write the formula for the compound formed...Ch. 3 - 3-44 Write the formula for the ionic compound...Ch. 3 - 3-45 Which formulas are not correct? For each that...Ch. 3 - 3-46 Which formulas are not correct? For each that...Ch. 3 - 3-47 Answer true or false. (a) The name of a...Ch. 3 - 3-48 Potassium chloride and potassium bicarbonate...Ch. 3 - Prob. 33PCh. 3 - 3-50 Name the polyatomic ion(s) in each compound....Ch. 3 - 3-51 Write the formulas for the ions present in...Ch. 3 - Prob. 36PCh. 3 - 3-53 Write formulas for the following ionic...Ch. 3 - 3-54 Write formulas for the following ionic...Ch. 3 - Prob. 39PCh. 3 - 3-56 How many covalent bonds are normally formed...Ch. 3 - 3-57 What is: (a) A single bond? (b) A double...Ch. 3 - 3-58 In Section 2-3B, we saw that there are seven...Ch. 3 - Prob. 43PCh. 3 - Prob. 44PCh. 3 - Prob. 45PCh. 3 - Prob. 46PCh. 3 - 3-63 What is the difference between (a) a bromine...Ch. 3 - 3-64 Acetylene (C2H2), hydrogen cyanide (HCN), and...Ch. 3 - Prob. 49PCh. 3 - 3-66 Why can’t second-row elements have more than...Ch. 3 - 3-67 Why does nitrogen have three bonds and one...Ch. 3 - 3-68 Draw a Lewis structure of a covalent compound...Ch. 3 - Prob. 53PCh. 3 - 3-70 Draw a Lewis structure of a covalent compound...Ch. 3 - Prob. 55PCh. 3 - Prob. 56PCh. 3 - Prob. 57PCh. 3 - 3-74 Answer true or false. (a) A binary covalent...Ch. 3 - Prob. 59PCh. 3 - Prob. 60PCh. 3 - 3-77 Ozone, O3, is an unstable blue gas with a...Ch. 3 - 3-78 Nitrous oxide, N20, laughing gas, is a...Ch. 3 - 3-79 Answer true or false. (a) The letters VSEPR...Ch. 3 - Prob. 64PCh. 3 - Prob. 65PCh. 3 - 3-82 Hydrogen and nitrogen combine in different...Ch. 3 - Prob. 67PCh. 3 - Prob. 68PCh. 3 - Prob. 69PCh. 3 - Prob. 70PCh. 3 - 3-87 Consider the molecule boron trffluoride, BF3....Ch. 3 - Prob. 72PCh. 3 - 3-89 Is it possible for a molecule to have no...Ch. 3 - Prob. 74PCh. 3 - Prob. 75PCh. 3 - Prob. 76PCh. 3 - Prob. 77PCh. 3 - Prob. 78PCh. 3 - Prob. 79PCh. 3 - Prob. 80PCh. 3 - Prob. 81PCh. 3 - Prob. 82PCh. 3 - 3-99 Knowing what you do about covalent bonding in...Ch. 3 - Prob. 84PCh. 3 - Prob. 85PCh. 3 - Prob. 86PCh. 3 - Prob. 87PCh. 3 - Prob. 88PCh. 3 - 3-105 Consider the structure of Vitamin E shown...Ch. 3 - 3-106 Consider the structure of Penicillin G shown...Ch. 3 - 3-107 Ephedrine, a molecule at one time found in...Ch. 3 - Prob. 92PCh. 3 - 3-109 Until several years ago, the two...Ch. 3 - 3-110 Name and write the formula for the fluorine...Ch. 3 - Prob. 95PCh. 3 - Prob. 96PCh. 3 - Prob. 97PCh. 3 - Prob. 98PCh. 3 - Prob. 99PCh. 3 - Prob. 100PCh. 3 - Prob. 101PCh. 3 - Prob. 102PCh. 3 - 3-119 Perchloroethylene, which is a liquid at room...Ch. 3 - 3-120 Vinyl chloride is the starting material for...Ch. 3 - 3-121 Tetrafluoroethylene is the starting material...Ch. 3 - 3-122 Some of the following structural formulas...Ch. 3 - 3-123 Sodium borohydride, NaBH4, has found wide...Ch. 3 - Prob. 108PCh. 3 - Prob. 109PCh. 3 - Prob. 110PCh. 3 - Prob. 111PCh. 3 - Prob. 112PCh. 3 - Consider the structure of Fluoxetine (or Prozac)...Ch. 3 - Consider the structure of lipoic acid shown below,...Ch. 3 - Prob. 115P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- It is not unexpected that the methoxyl substituent on a cyclohexane ring prefers to adopt the equatorial conformation. OMe H A G₂ = +0.6 kcal/mol OMe What is unexpected is that the closely related 2-methoxytetrahydropyran prefers the axial conformation: H H OMe OMe A Gp=-0.6 kcal/mol Methoxy: CH3O group Please be specific and clearly write the reason why this is observed. This effect that provides stabilization of the axial OCH 3 group in this molecule is called the anomeric effect. [Recall in the way of example, the staggered conformer of ethane is more stable than eclipsed owing to bonding MO interacting with anti-bonding MO...]arrow_forward206 Pb 82 Express your answers as integers. Enter your answers separated by a comma. ▸ View Available Hint(s) VAΣ ΜΕ ΑΣΦ Np, N₁ = 82,126 Submit Previous Answers ? protons, neutronsarrow_forwardPlease draw the inverted chair forms of the products for the two equilibrium reactions shown below. Circle the equilibrium reaction that would have a AG = 0, i.e., the relative energy of the reactant (to the left of the equilibrium arrows) equals the relative energy of the product? [No requirement to show or do calculations.] CH3 CH3 HH CH3 1 -CH3arrow_forward
- 5. Please consider the Newman projection of tartaric acid drawn below as an eclipsed conformer (1). Please draw the most stable conformer and two intermediate energy conformers noting that staggered conformers are lower in energy than eclipsed forms even if the staggered conformers have gauche relationships between groups. [Draw the substituents H and OH on the front carbons and H, OH and CO₂H on the back carbons based on staggered forms. -CO₂H is larger than -OH.] OH COH ICOOH COOH COOH 1 2 COOH COOH 3 4 Staggered Staggered Staggered (most stable) Indicate the number of each conformer above (1, 2, 3 and 4) that corresponds to the relative energies below. Ref=0 Rotation 6. (60 points) a. Are compounds 1 and 2 below enantiomers, diastereomers or identical? OH OH HO HO LOH HO HO OH 2 OH OH b. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 3.arrow_forwardThe plutonium isotope with 144 neutrons Enter the chemical symbol of the isotope.arrow_forwardThe mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produced 26.1 gg of sodium upon decomposition. How much fluorine was formed?arrow_forward
- 32S 16 Enter your answers numerically separated by a comma. Np. Nn = 跖 ΟΙ ΑΣΦ Submit Request Answer ? protons, neutronsarrow_forward2. Which dimethylcyclohexane compounds shown below exhibit symmetry and therefore are not chiral and would not rotate plane polarized light. 1 CH3 CH CH3 CH3 2 3 CH3arrow_forwardDon't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY