
Concept explainers
(a)
Interpretation:
Molecular formula for unknown compound has to be determined.
Concept Introduction:
Here,
(a)
Answer to Problem 3.9E
Mass of
Explanation of Solution
Conversion factor to convert
Rearrange equation (1) to determine value of
Substitute
Number of moles of nitrogen present in
Number of moles of hydrogen gas present in
Ratio of nitrogen to hydrogen can be calculated as follows:
Therefore formula unit is
Total molar mass of gas can be calculated as follows:
Therefore, number of formula unit of
(b)
Interpretation:
Lewis structure of
Concept Introduction:
Lewis structure represents covalent bonds and describes valence electrons configuration of atoms. The covalent bonds are depicted by lines and unshared electron pairs by pairs of dots. The sequence to write Lewis structure of some molecule is given as follows:
- The central atom is identified and various other atoms are arranged around it. This central atom so chosen is often the least electronegative.
- Total valence electrons are estimated for each atom.
- single bond is first placed between each atom pair.
- The electrons left can be allocated as unshared electron pairs or as multiple bonds around
symbol of element to satisfy the octet (or duplet) for each atom. - Add charge on overall structure in case of polyatomic cation or anion.
(b)
Explanation of Solution
The molecule
Electronic configuration of
Electronic configuration of
Thus total valence electrons are sum of the valence electrons for each atom in
Skeleton structure
Hence, 4 electrons are allocated as 2 lone pairs on each nitrogen atom. The Lewis structure of
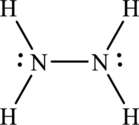
(c)
Interpretation:
Amount of nitrogen gas effused through small hole in
Concept Introduction:
Effusion is explained as movement of the gas molecule through small hole. Diffusion can be explained as one gas molecule mix with another gas molecule by random motion.
(c)
Answer to Problem 3.9E
Amount of nitrogen gas effused through small hole in
Explanation of Solution
The expression for molar masses of
Rearrange equation (3) to calculate amount of
Substitute
Hence amount of nitrogen gas effused through small hole in
Want to see more full solutions like this?
Chapter 3 Solutions
Chemical Principles: The Quest for Insight
- In intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?arrow_forwardThis thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward7. Draw the mechanism to describe the following transformation: Note: This is a base catalyzed reaction. So, the last steps must make [OH]- OH [OH]¯ OH Heat Oarrow_forward
- We are practicing calculating for making solutions. How would I calculate this?arrow_forwardBr. , H+ .OH Mg ether solvent H+, H₂O 17. Which one of the compounds below is the final product of the reaction sequence shown above? HO A HO HO OH D B OH HO OH C OH HO OH Earrow_forward8:57 PM Sun Jan 26 Content ← Explanation Page X Content X ALEKS Jade Nicol - Le A https://www-av C www-awa.aleks.com O States of Matter Understanding consequences of important physical properties of liquids ? QUESTION Liquid A is known to have a lower viscosity and lower surface tension than Liquid B. Use these facts to predict the result of each experiment in the table below, if you can. experiment Liquid A and Liquid B are each pumped through tubes with an inside diameter of 27.0 mm, and the pressures PA and PB needed to produce a steady flow of 2.4 mL/s are measured. 25.0 mL of Liquid A are poured into a beaker, and 25.0 mL of Liquid B are poured into an identical beaker. Stirrers in each beaker are connected to motors, and the forces FA and FB needed to stir each liquid at a constant rate are measured. predicted outcome OPA will be greater than PB OPA will be less than PB OPA will be equal to PB It's impossible to predict whether PA or PB will be greater without more information.…arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





