
(a)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
Alcohols undergo halogenation reaction to give halogenated product. Alcohols on halogenation gives halogenated product in which the hydroxyl group present in the alcohol is substituted by the halogen. Phosphorous trihalides are useful in producing
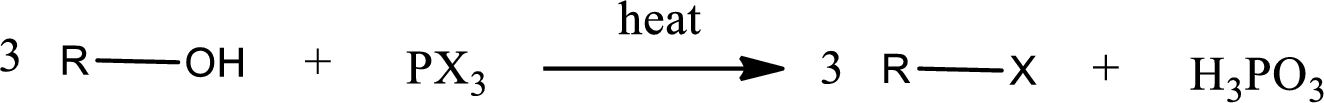
(b)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
Dehydration reaction is the loss of water from a single reactant. Alcohol undergoes dehydration reaction to form
(c)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
In
In organic chemistry, reduction reaction is referred to the number
Alcohols do undergo
(d)
Interpretation:
The structure of predominant organic product that is formed in the given reaction has to be drawn.
Concept Introduction:
Dehydration reaction is the loss of water from a single reactant. Alcohol undergoes dehydration reaction to form alkene. Sulfuric acid acts as a catalyst for hydration of alkene at room temperature. The same sulfuric acid acts as a dehydrating agent when treated with alcohol at high temperature. If the reaction is carried out at a lower temperature, the loss of water molecule takes place from two molecule of reactant. This results in the formation of ether. Primary alcohol when treated with sulfuric acid at lower temperature (
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Organic And Biological Chemistry
- -C = C - C - + Br₂ + I" -> -C-C-c -C = C -C- + Br² + I₂ -C=C Br I + Brū + Iz -7- C - C-C- I Br Mechanism; - C = c - c - + Br - Br > - C-c-c- Br -C-C-C- + 1 - - -Ċ-Ċ'-c' - Br Br Iarrow_forwardWrite the mechanism of the esterification reaction (please show the mechanism included line pairs and arrows)arrow_forwardHow do I break down the reaction shown on the chalkboard and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanismarrow_forward
- ¿Qué the product is obtained from tetraethoxypropano and hidrazina?. Indicate the reason why the corresponding dial is used.arrow_forwardIf CH3COCH2CH(OCH3)2 is reacted with hydrazine, two isomeric products are formed. Indicate their structures and the major product.arrow_forwardIs it possible to obtain addition derivatives to nitrogen in position 2 of pyrazoles by reaction with electrophilic agents? Reason for this.arrow_forward
- Starting from 1,3-dicarbonyl derivatives to obtain isooxazoles and isothiazoles. Indicate whether synthetic methods exist.arrow_forwardIn the synthesis of benzotriazole, adding NaNO2 heats the solution. State the reason.arrow_forwardIndicate the products obtained by treating benzotriazole with dimethyl sulfate or methyl iodide in a basic medium.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





