
Concept explainers
(a)
Interpretation:
The predominant organic product that is formed in the given reaction has to be written.
Concept Introduction:
Alcohol is an organic compound that has hydroxyl as its
Alkenes are compounds that contain a double bond between carbon atoms. When alkenes undergo hydration in presence of sulfuric acid as catalyst, an alcohol is formed as product. The major product formed in case of unsymmetrical alkene is found by using Markovnikov’s rule. The general scheme for hydration of alkene can be given as,
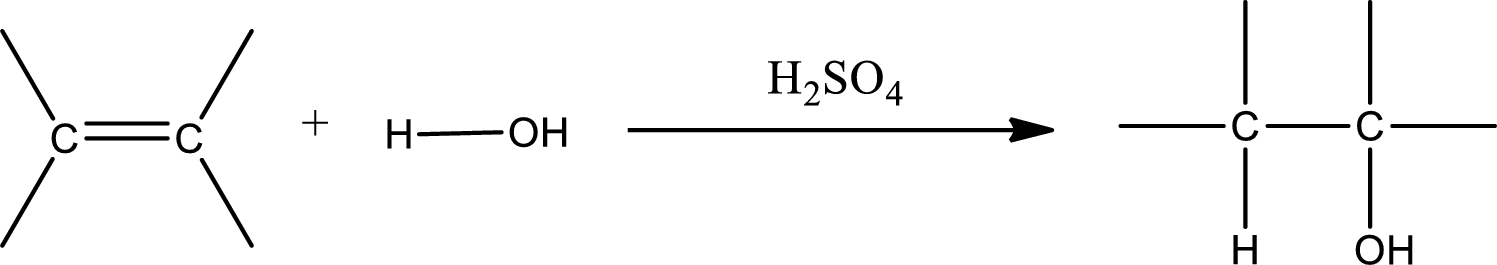
(b)
Interpretation:
The predominant organic product that is formed in the given reaction has to be written.
Concept Introduction:
Alcohol is an organic compound that has hydroxyl as its functional group. Alcohols contain both nonpolar and polar groups in it. Hydroxyl group is the polar group and the alkyl group is the nonpolar group. Physical properties of alcohol depend on which of the two groups dominate. Alcohols can be prepared in laboratory by hydration of alkenes and reduction of carbonyl compounds.
Double bond between a carbon atom and oxygen atom means that the compound is a carbonyl compound. Addition of hydrogen to this carbonyl group leads to the formation of alcohol. When hydrogen is added to the carbonyl, the oxygen of the carbonyl is converted into hydroxyl group. A scheme for the addition of hydrogen to the carbonyl group can be given as shown below,
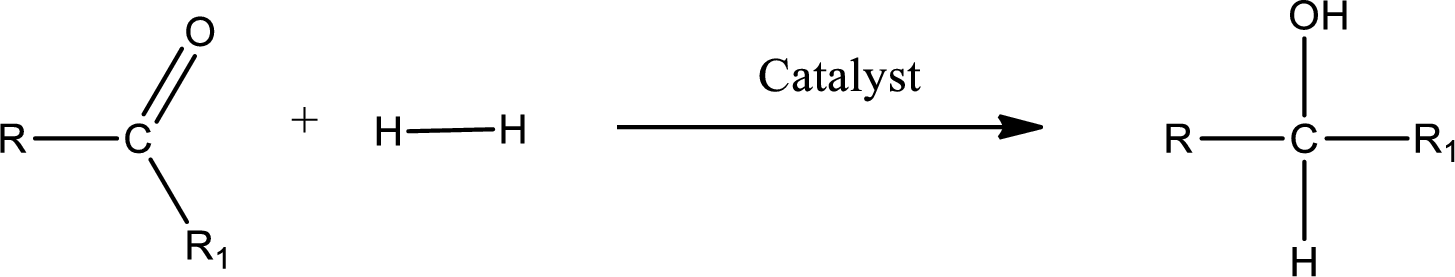
(c)
Interpretation:
The predominant organic product that is formed in the given reaction has to be written.
Concept Introduction:
Alcohol is an organic compound that has hydroxyl as its functional group. Alcohols contain both nonpolar and polar groups in it. Hydroxyl group is the polar group and the alkyl group is the nonpolar group. Physical properties of alcohol depend on which of the two groups dominate. Alcohols can be prepared in laboratory by hydration of alkenes and reduction of carbonyl compounds.
Double bond between a carbon atom and oxygen atom means that the compound is a carbonyl compound. Addition of hydrogen to this carbonyl group leads to the formation of alcohol. When hydrogen is added to the carbonyl, the oxygen of the carbonyl is converted into hydroxyl group. A scheme for the addition of hydrogen to the carbonyl group can be given as shown below,
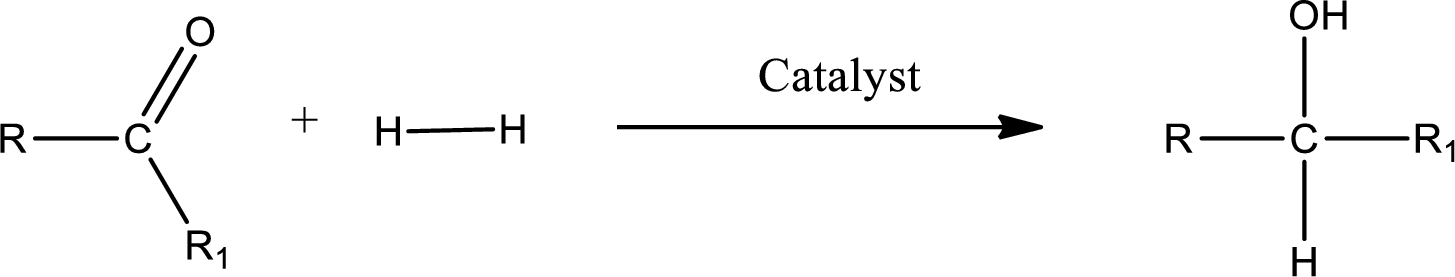
(d)
Interpretation:
The predominant organic product that is formed in the given reaction has to be written.
Concept Introduction:
Alcohol is an organic compound that has hydroxyl as its functional group. Alcohols contain both nonpolar and polar groups in it. Hydroxyl group is the polar group and the alkyl group is the nonpolar group. Physical properties of alcohol depend on which of the two groups dominate. Alcohols can be prepared in laboratory by hydration of alkenes and reduction of carbonyl compounds.
Alkenes are compounds that contain a double bond between carbon atoms. When alkenes undergo hydration in presence of sulfuric acid as catalyst, an alcohol is formed as product. The major product formed in case of unsymmetrical alkene is found by using Markovnikov’s rule. The general scheme for hydration of alkene can be given as,
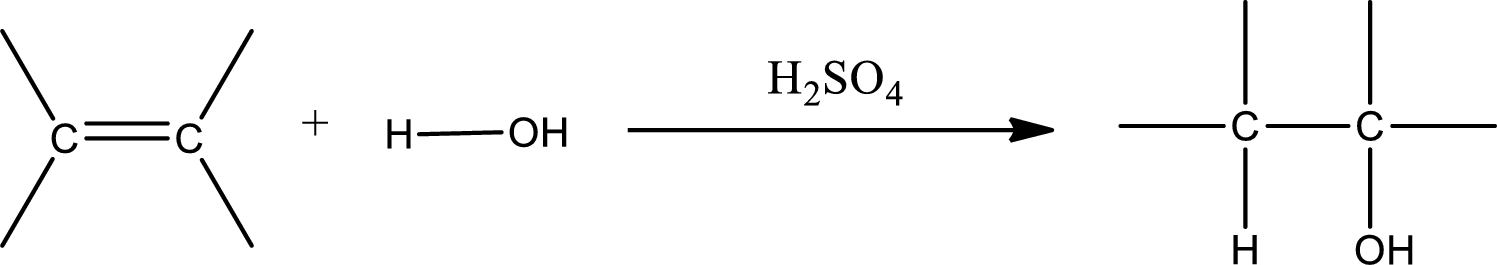
Trending nowThis is a popular solution!

Chapter 3 Solutions
Organic And Biological Chemistry
- Indicate the product that is obtained if the benzotriazol reacts with dimethyl sulfate.arrow_forwardIndicate how to obtain 2-metilbencimidazol from 1,2-diaminobenzene.arrow_forwardbreak down both reactions shown and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanism.arrow_forward
- Indicate how from 1,2-diaminobenzene to obtain 1-metilbenzotriazol.arrow_forward-C = C - C - + Br₂ + I" -> -C-C-c -C = C -C- + Br² + I₂ -C=C Br I + Brū + Iz -7- C - C-C- I Br Mechanism; - C = c - c - + Br - Br > - C-c-c- Br -C-C-C- + 1 - - -Ċ-Ċ'-c' - Br Br Iarrow_forwardWrite the mechanism of the esterification reaction (please show the mechanism included line pairs and arrows)arrow_forward
- How do I break down the reaction shown on the chalkboard and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanismarrow_forward¿Qué the product is obtained from tetraethoxypropano and hidrazina?. Indicate the reason why the corresponding dial is used.arrow_forwardIf CH3COCH2CH(OCH3)2 is reacted with hydrazine, two isomeric products are formed. Indicate their structures and the major product.arrow_forward
- Is it possible to obtain addition derivatives to nitrogen in position 2 of pyrazoles by reaction with electrophilic agents? Reason for this.arrow_forwardStarting from 1,3-dicarbonyl derivatives to obtain isooxazoles and isothiazoles. Indicate whether synthetic methods exist.arrow_forwardIn the synthesis of benzotriazole, adding NaNO2 heats the solution. State the reason.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





