
Concept explainers
How many protons, neutrons, and electrons are present in atoms with the following characteristics?
- a.
atomic number = 7, mass number = 15 - b. atomic number = 20, mass number = 40
- c. Z = 11, A = 23
- d. Z = 35, A = 79
(a)
Interpretation:
The number of protons, neutrons and electrons present in the atom that has atomic number of 7 and mass number of 15 has to be given.
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
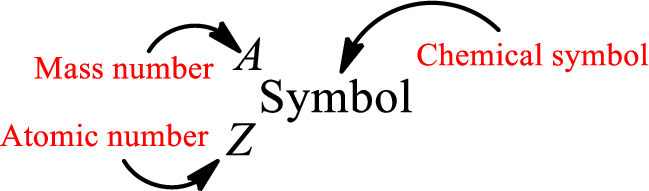
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given atom is said to have atomic number as 7 and mass number as 15. The number of protons, electrons and neutrons can be found as shown below,
Atomic number is given as 7 and mass number is given as 15.
Therefore, the number of protons is 7, number of electrons is 7, and number of neutrons is 8.
The number of protons, electrons and neutrons present in the atom is given.
(b)
Interpretation:
The number of protons, neutrons and electrons present in the atom that has atomic number of 20 and mass number of 40 has to be given.
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
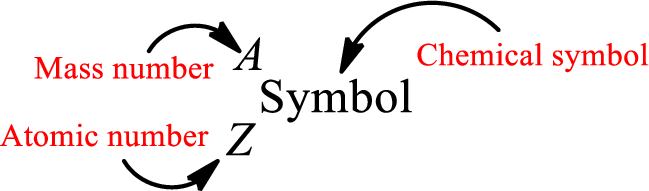
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given atom is said to have atomic number as 20 and mass number as 40. The number of protons, electrons and neutrons can be found as shown below,
Atomic number is given as 20 and mass number is given as 40.
Therefore, the number of protons is 20, number of electrons is 20, and number of neutrons is 20.
The number of protons, electrons and neutrons present in the atom is given.
(c)
Interpretation:
The number of protons, neutrons and electrons present in the atom which has Z as 11 and A as 23 has to be given.
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
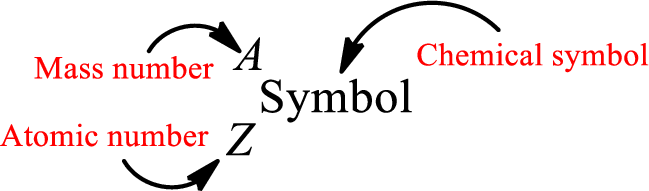
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given atom is said to have Z as 11 and A as 23. The number of protons, electrons and neutrons can be found as shown below,
Atomic number is given as 11 and mass number is given as 23.
Therefore, the number of protons is 11, number of electrons is 11, and number of neutrons is 12.
The number of protons, electrons and neutrons present in the atom is given.
(d)
Interpretation:
The number of protons, neutrons and electrons present in the atom which has Z as 35 and A as 79 has to be given.
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
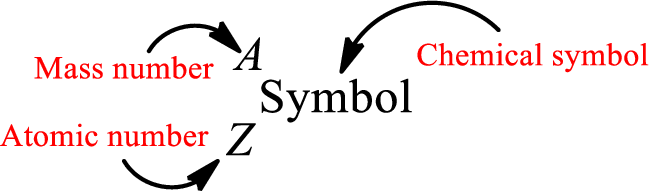
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given atom is said to have Z as 35 and A as 79. The number of protons, electrons and neutrons can be found as shown below,
Atomic number is given as 35 and mass number is given as 79.
Therefore, the number of protons is 35, number of electrons is 35, and number of neutrons is 34.
The number of protons, electrons and neutrons present in the atom is given.
Want to see more full solutions like this?
Chapter 3 Solutions
General, Organic, and Biological Chemistry
- The Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning  Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





