
(a)
Interpretation:
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
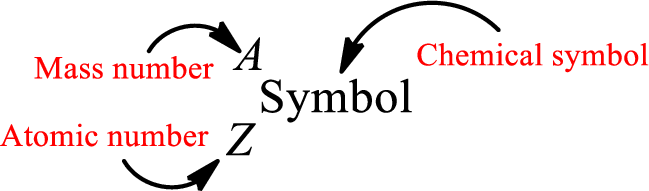
An element is a pure substance that cannot be broken by ordinary
(a)
Explanation of Solution
For
The atomic number is given as 7. The element is found to be nitrogen. Mass number given for nitrogen is given as 13. The number of protons present in it is also 7 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 6.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 13.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 20.
For
The atomic number is given as 6. The element is found to be carbon. Mass number given for carbon is given as 13. The number of protons present in it is also 6 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 7.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 13.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 19.
On comparing both the pair of atoms it is found that they contain same number of nucleons only.
(b)
Interpretation:
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
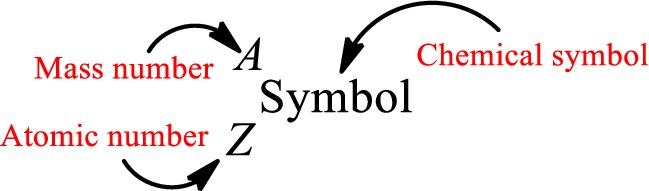
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(b)
Explanation of Solution
For
The atomic number is given as 17. The element is found to be chlorine. Mass number given for chlorine is given as 37. The number of protons present in it is also 17 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 20.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 37.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 54.
For
The atomic number is given as 18. The element is found to be argon. Mass number given for argon is given as 36. The number of protons present in it is also 18 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 18.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 36.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 54.
On comparing both the pair of atoms it is found that they contain same number of subatomic particles in them.
(c)
Interpretation:
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
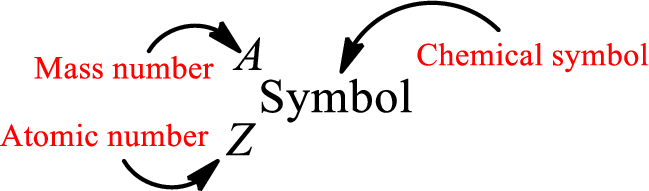
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(c)
Explanation of Solution
For
The atomic number is given as 17. The element is found to be chlorine. Mass number given for chlorine is given as 35. The number of protons present in it is also 17 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 18.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 35.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 52.
For
The atomic number is given as 17. The element is found to be chlorine. Mass number given for chlorine is given as 37. The number of protons present in it is also 17 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 20.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 37.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 54.
On comparing both the pair of atoms it is found that they contain same number of protons.
(d)
Interpretation:
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
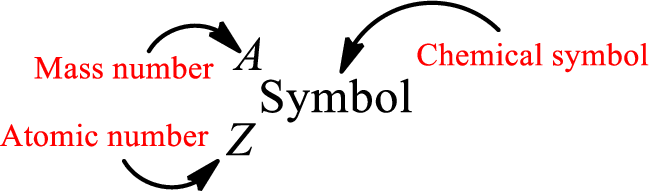
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(d)
Explanation of Solution
For
The atomic number is given as 8. The element is found to be oxygen. Mass number given for oxygen is given as 18. The number of protons present in it is also 8 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 10.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 18.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 26.
For
The atomic number is given as 9. The element is found to be fluorine. Mass number given for fluorine is given as 19. The number of protons present in it is also 17 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 10.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 19.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 28.
On comparing both the pair of atoms it is found that they contain same number of neutrons.
Want to see more full solutions like this?
Chapter 3 Solutions
General, Organic, and Biological Chemistry
- Predict the major products of this organic reaction: H OH 1. LiAlH4 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. G C टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C-C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 CI MgCl ? Will the first product that forms in this reaction create a new CC bond? Yes No MgBr ? Will the first product that forms in this reaction create a new CC bond? Yes No G टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forward
- Predict the major products of this organic reaction: OH NaBH4 H ? CH3OH Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. ☐ : Sarrow_forwardPredict the major products of this organic reaction: 1. LIAIHA 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. X : ☐arrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forward
- As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forwardgive example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward
- 2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





