
Concept explainers
(a)
Interpretation:
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
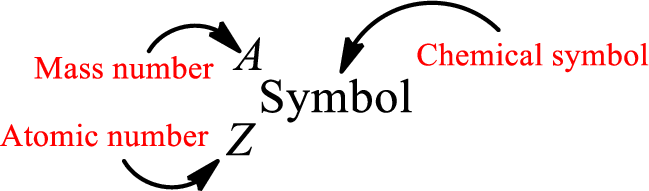
An element is a pure substance that cannot be broken by ordinary
(a)
Explanation of Solution
For
The atomic number is given as 18. The element is found to be argon. Mass number given for argon is given as 40. The number of protons present in it is also 18 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 22.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 40.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 58.
For
The atomic number is given as 20. The element is found to be calcium. Mass number given for calcium is given as 40. The number of protons present in it is also 20 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 20.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 40.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 60.
On comparing both the pair of atoms it is found that they contain same number of nucleons only.
(b)
Interpretation:
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
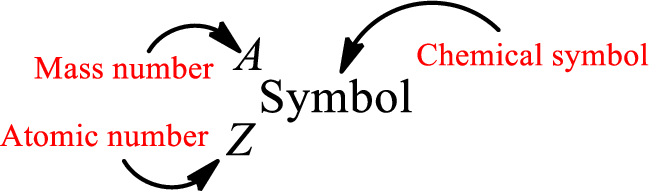
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(b)
Explanation of Solution
For
The atomic number is given as 7. The element is found to be nitrogen. Mass number given for nitrogen is given as 14. The number of protons present in it is also 7 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 7.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 14.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 21.
For
The atomic number is given as 7. The element is found to be nitrogen. Mass number given for nitrogen is given as 15. The number of protons present in it is also 7 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 8.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 15.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 22.
On comparing both the pair of atoms it is found that they contain same number of protons.
(c)
Interpretation:
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
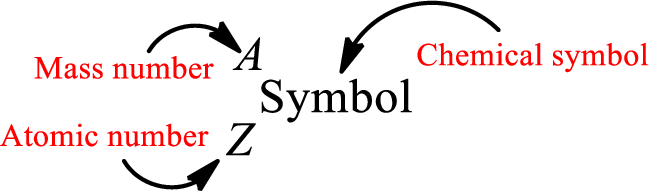
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(c)
Explanation of Solution
For
The atomic number is given as 6. The element is found to be carbon. Mass number given for carbon is given as 14. The number of protons present in it is also 6 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 8.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 14.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 22.
For
The atomic number is given as 7. The element is found to be nitrogen. Mass number given for nitrogen is given as 15. The number of protons present in it is also 7 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 8.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 15.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 22.
On comparing both the pair of atoms it is found that they contain same number of neutrons.
(d)
Interpretation:
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
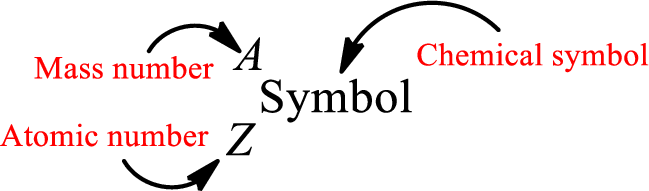
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(d)
Explanation of Solution
For
The atomic number is given as 18. The element is found to be argon. Mass number given for argon is given as 38. The number of protons present in it is also 18 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 20.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 38.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 56.
For
The atomic number is given as 19. The element is found to be potassium. Mass number given for potassium is given as 39. The number of protons present in it is also 19 as atomic number is the number of protons or electrons. Mass number of the sum of protons and neutrons present in the atom.
The number of neutrons can be identified by finding the difference between mass number and atomic number. This gives the number of neutrons to be 20.
The total number of nucleons is same as that of the mass number. This means the total number of nucleons is 39.
The total number of subatomic particle present in the given atom is the sum of mass number and atomic number. This means the total number of subatomic particle is 58.
On comparing both the pair of atoms it is found that they contain same number of neutrons.
Want to see more full solutions like this?
Chapter 3 Solutions
General, Organic, and Biological Chemistry
- What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forwardCan I please get help with this?arrow_forward
- Can I please get help with this?arrow_forwardUse the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forward
- What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





