
(a)
Interpretation:
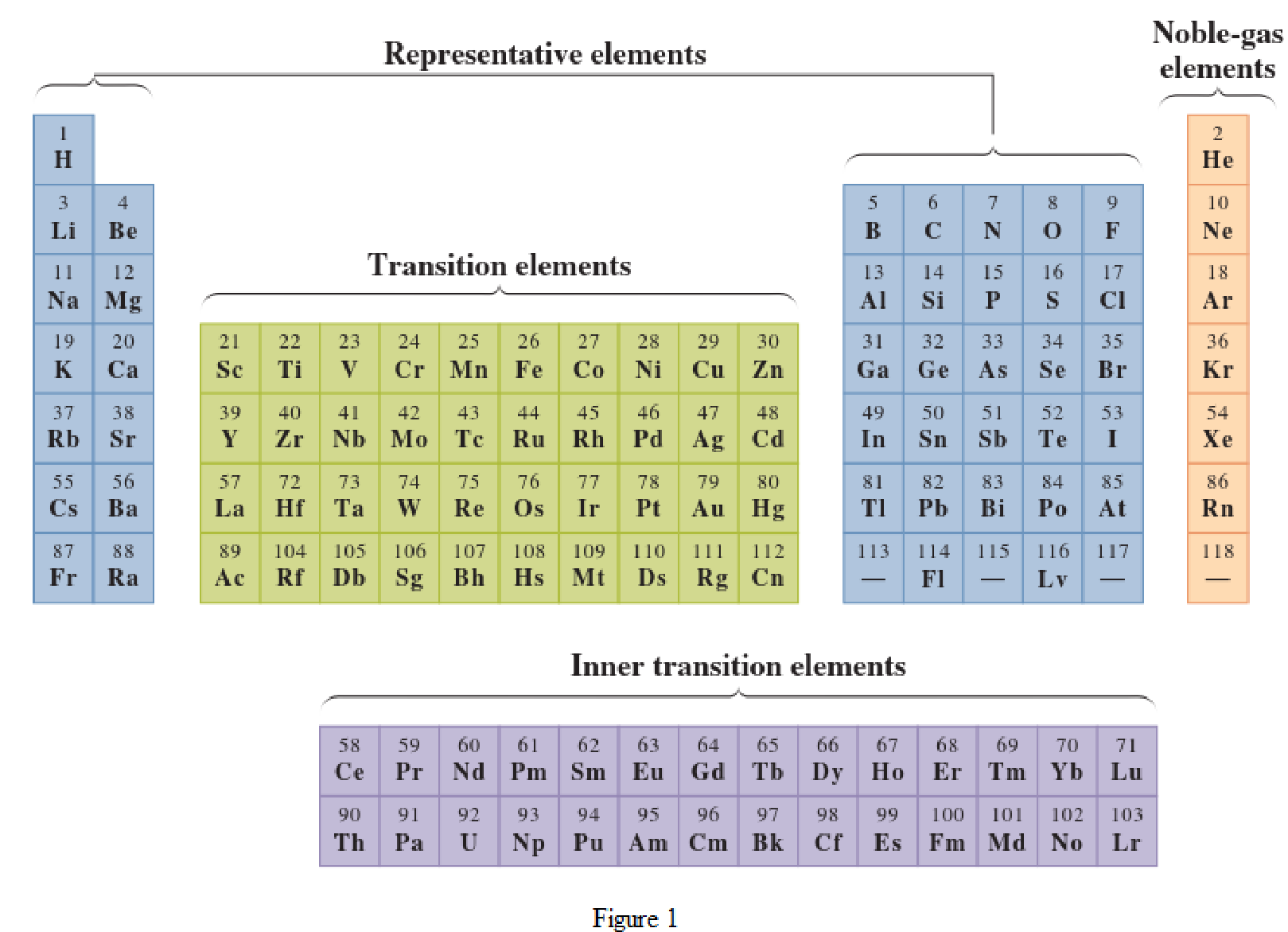
In the given periodic table, how many elements those are highlighted which represent inner
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
(b)
Interpretation:
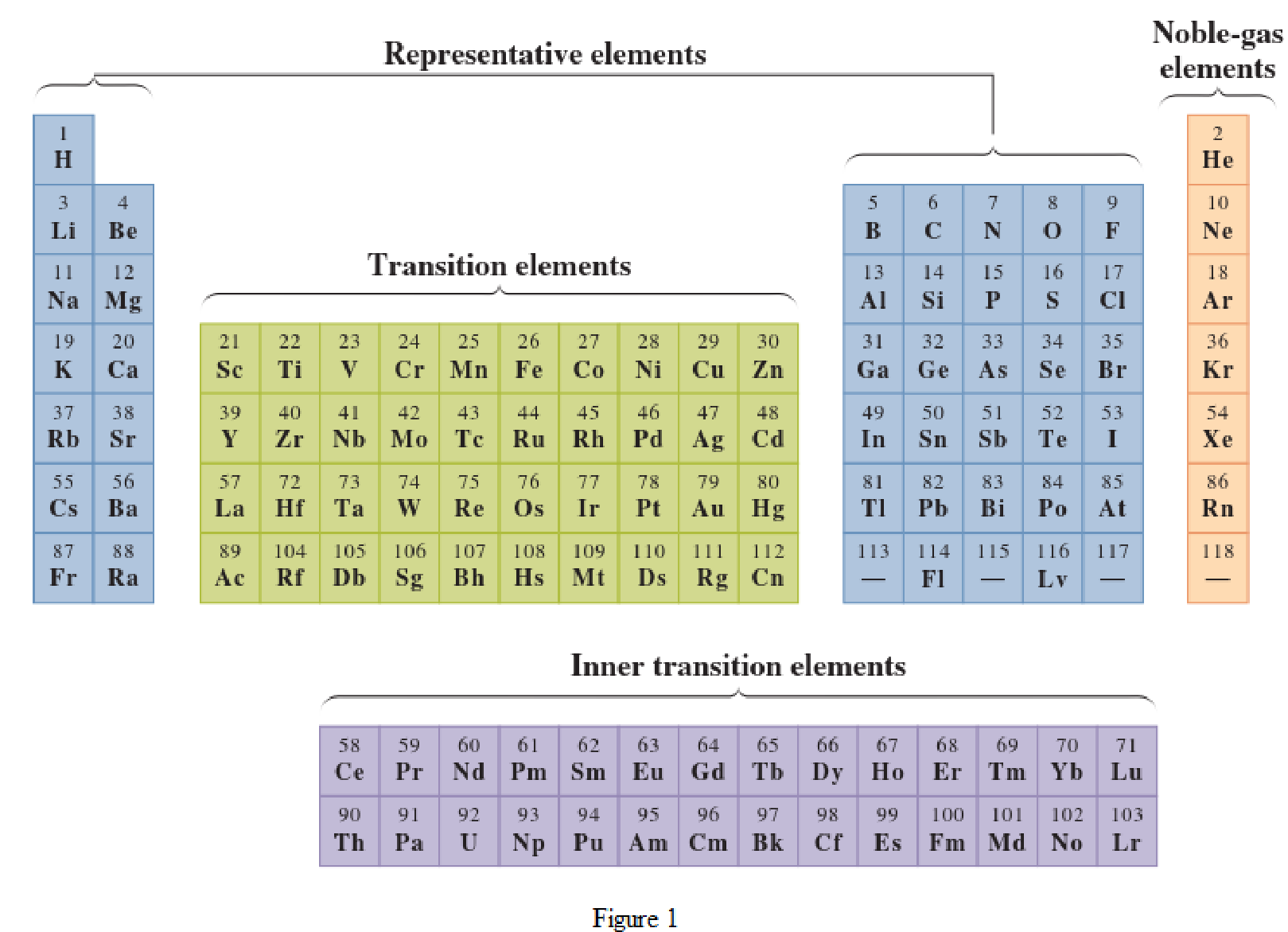
In the given periodic table, how many elements those are highlighted which represent transition have to be determined.
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
(c)
Interpretation:
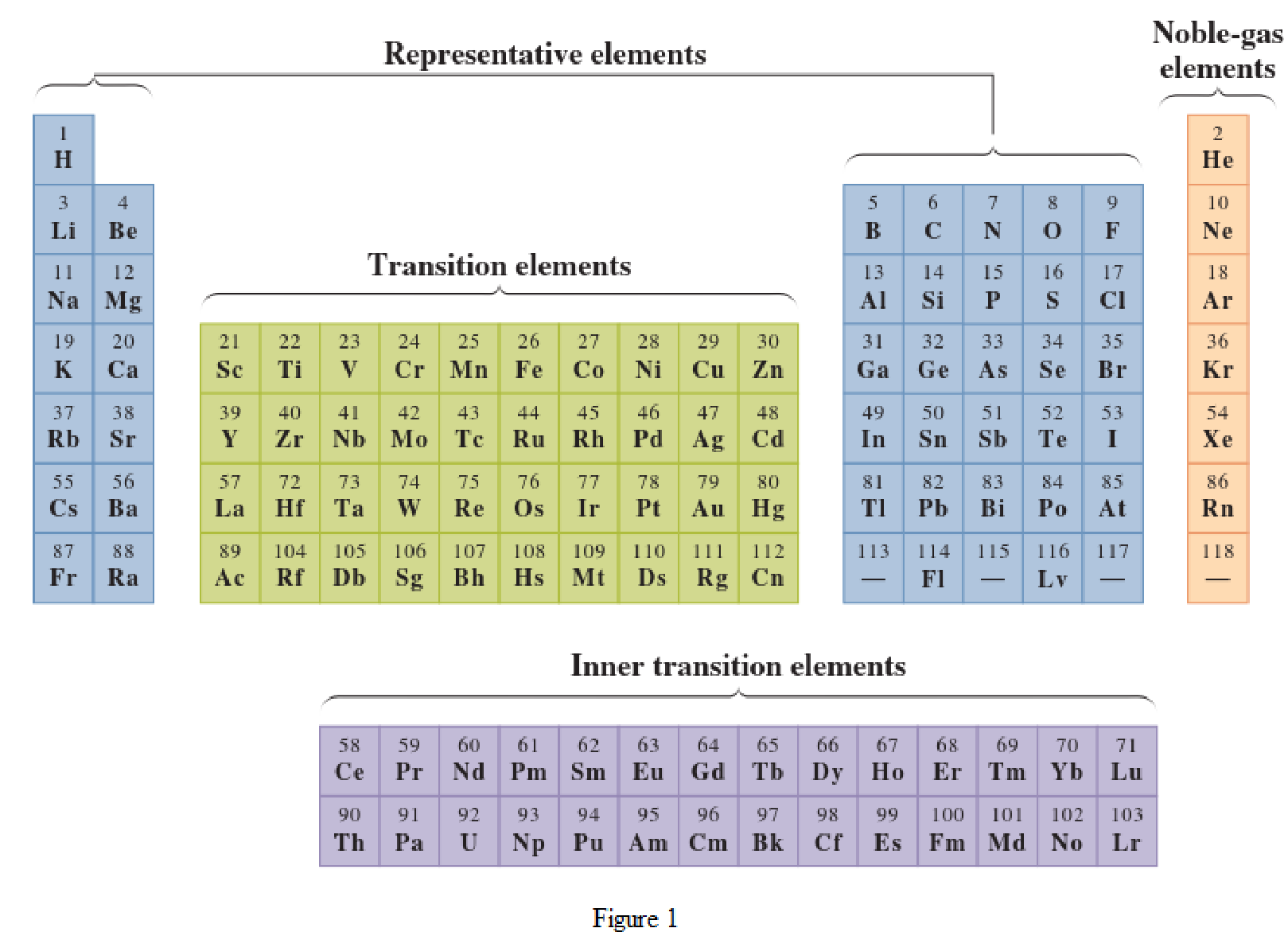
In the given periodic table, how many elements those are highlighted which represent metallic representative elements have to be determined.
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
(d)
Interpretation:
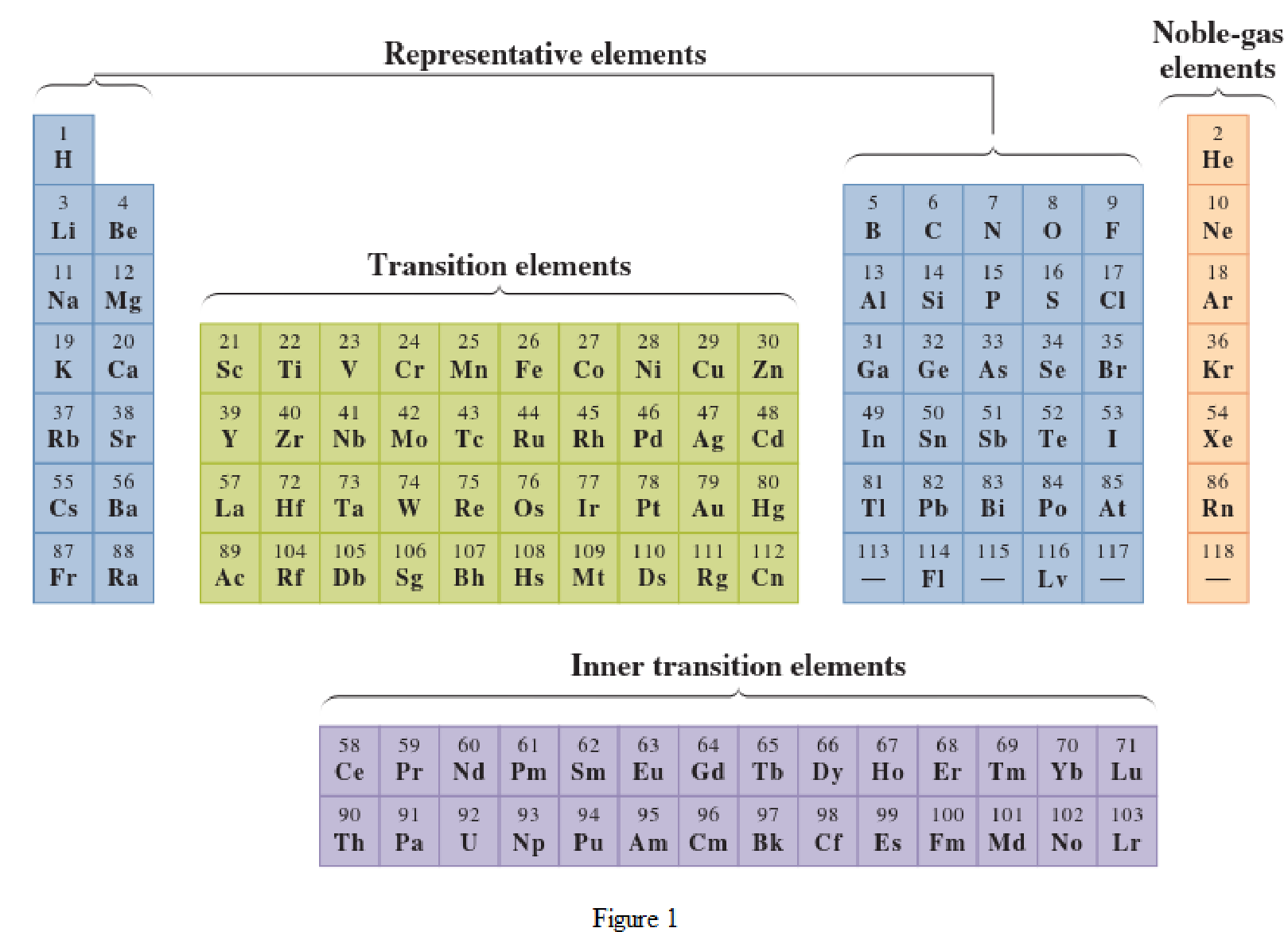
In the given periodic table, how many elements those are highlighted which represent nonmetals have to be determined.
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
General, Organic, and Biological Chemistry
- Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





