
(a)
Interpretation:
The given alcohol is classified as what type of alcohol has to be identified.
Concept Introduction:
Alcohols are classified as primary, secondary, or tertiary depending upon the number of carbon atoms that is bonded to the carbon atom bearing hydroxyl group.
Primary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to only one other carbon atom.
Secondary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to two other carbon atoms.
Tertiary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to three other carbon atoms.
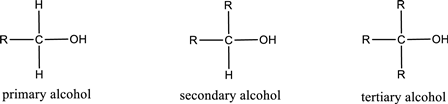
Primary is denoted as
(b)
Interpretation:
The given alcohol is classified as what type of alcohol has to be identified.
Concept Introduction:
Alcohols are classified as primary, secondary, or tertiary depending upon the number of carbon atoms that is bonded to the carbon atom bearing hydroxyl group.
Primary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to only one other carbon atom.
Secondary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to two other carbon atoms.
Tertiary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to three other carbon atoms.
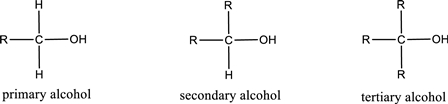
Primary is denoted as
(c)
Interpretation:
The given alcohol is classified as what type of alcohol has to be identified.
Concept Introduction:
Alcohols are classified as primary, secondary, or tertiary depending upon the number of carbon atoms that is bonded to the carbon atom bearing hydroxyl group.
Primary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to only one other carbon atom.
Secondary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to two other carbon atoms.
Tertiary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to three other carbon atoms.
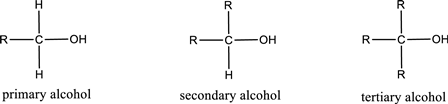
Primary is denoted as
(d)
Interpretation:
The given alcohol is classified as what type of alcohol has to be identified.
Concept Introduction:
Alcohols are classified as primary, secondary, or tertiary depending upon the number of carbon atoms that is bonded to the carbon atom bearing hydroxyl group.
Primary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to only one other carbon atom.
Secondary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to two other carbon atoms.
Tertiary alcohol is the one in which the carbon atom bearing the hydroxyl group is bonded to three other carbon atoms.
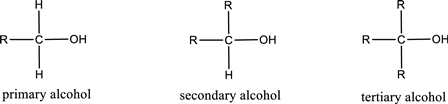
Primary is denoted as
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





