
Identify the
amide, and
a. 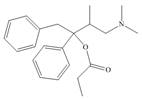 c.
c. 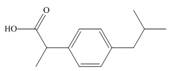 e.
e. 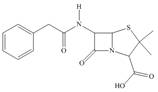
Darvon ibuprofen penicillin G
(analgesic) (analgesic) (an antibiotic)
b. 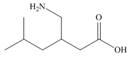 d.
d. 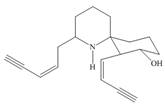 f.
f. 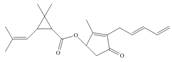
pregabalin histrionicotoxin pyrethrin I
trade name Lyrica (poison secreted by a (potent insecticide
(used in treating chronic south American frog) from chrysanthemum)
Pain)
(a)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.23P
The functional groups present in Darvon are shown below.
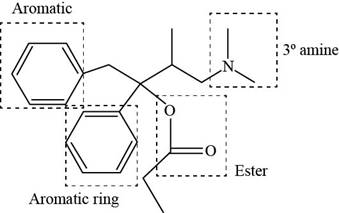
Explanation of Solution
The given compound is,
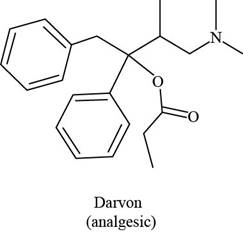
Figure 1
The functional groups present in Darvon are amine, ester and aryl group.
The structure of Darvon that shows the labeling of all functional groups is shown below.

Figure 2
The functional groups present in Darvon and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(b)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.23P
The functional groups present in pregabalin are shown below.
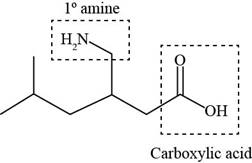
Explanation of Solution
The given structure is,
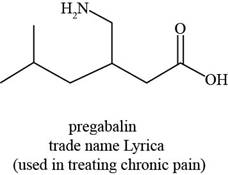
Figure 3
The functional groups present in pregabalin are amine, and carboxylic acid.
The structure of pregabalin that shows the classification of all functional group is shown below.

Figure 4
The functional groups present in pregabalin and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(c)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.23P
The functional groups present in ibuprofen are shown below.
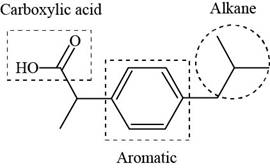
Explanation of Solution
The given structure is,
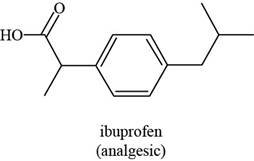
Figure 5
The functional groups present in ibuprofen are carboxylic acid, alkane and aryl.
The structure of ibuprofen that shows the classification of all functional group is shown below.

Figure 6
The functional groups present in ibuprofen and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(d)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.23P
The functional groups present in histrionicotoxin are shown below.
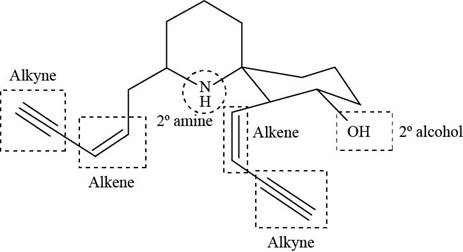
Explanation of Solution
The given structure is,
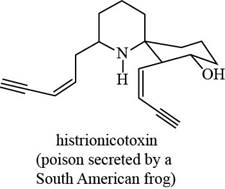
Figure 7
The functional groups present in histrionicotoxin are alkene, alkyne and alcohol. The structure of histrionicotoxin that shows the labeling of all functional group is shown below.

Figure 8
The functional groups present in histrionicotoxin and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(e)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.23P
The functional groups present in penicillin G are shown below.
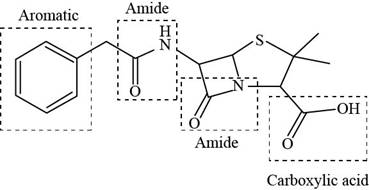
Explanation of Solution
The given structure is,
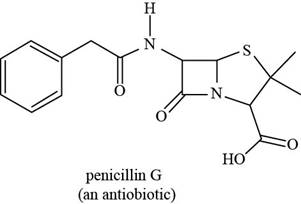
Figure 9
The functional groups present in penicillin G are carboxylic acid, amide, and aryl.
The structure of penicillin G that shows the labeling of all functional group is shown below.

Figure 10
The functional groups present in penicillin G and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(f)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.23P
The functional groups present in pyrethrin I are shown below.
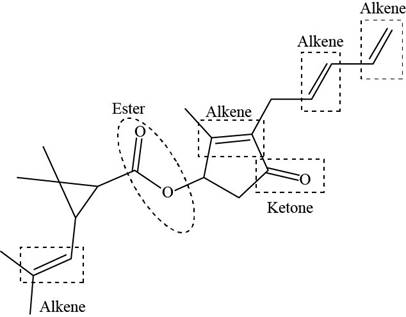
Explanation of Solution
The given structure is,
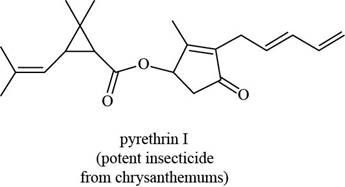
Figure 11
The functional groups present in pyrethrin I are ester, ketone, and alkene.
The structure of pyrethrin I that shows the labeling of all functional group is shown below.

Figure 12
The functional groups present in pyrethrin I and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry
- Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forward
- Can you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.arrow_forwardPart 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff: Ag₂ CO3 = 2 Ag+ caq) + co} (aq) ksp = 8.10 × 10-12 Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5 a) which salt will precipitate first? (b) What % of the first anion precipitated will remain in the solution. by the time the second anion starts to precipitate? (c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate explanation per answerarrow_forwardPart 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet. water and benzene. What is the formal concentration of butanoic acid in each phase when 0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene 100 mL of a) at pit 5.00 b) at pH 9.00arrow_forward
- Calculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





