
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 3.50P
By using only electron density arguments, determine whether the following reactions will
occur.
a.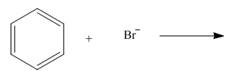 d.
d. 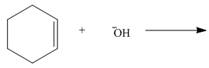
b. ![]() e.
e. ![]()
c. 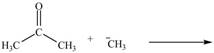
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the major products of this organic reaction:
HBr (1 equiv)
Δ
?
Some important notes:
• Draw the major product, or products, of this reaction in the drawing area below.
• You can draw the products in any arrangement you like.
• Pay careful attention to the reaction conditions, and only include the major products.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers.
• Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions.
Explanation
Check
X
©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy
For the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges.
:ÖH
Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the +
and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.
Using the table of Reactants and Products provided in the Hints section, provide the major product
(with the correct stereochemistry when applicable) for questions below by selecting the letter that
corresponds to the exact chemical structures for the possible product.
OH conc Hydrochloric
acid
40°C Temp
A/
Chapter 3 Solutions
Organic Chemistry
Ch. 3 - Prob. 3.1PCh. 3 - Prob. 3.2PCh. 3 - Draw the structure of a compound fitting each...Ch. 3 - Draw structures that fit each description and name...Ch. 3 - What types of intermolecular forces are present in...Ch. 3 - Prob. 3.6PCh. 3 - Explain why the boiling point of propanamide, is...Ch. 3 - Predict which compound in each pair has the higher...Ch. 3 - Prob. 3.9PCh. 3 - Prob. 3.10P
Ch. 3 - a Label the hydrophobic and hydrophilic portions...Ch. 3 - Prob. 3.12PCh. 3 - Prob. 3.13PCh. 3 - Prob. 3.14PCh. 3 - Prob. 3.15PCh. 3 - Nonactin and valinomycin each contain only two...Ch. 3 - Prob. 3.17PCh. 3 - Problem 3.26 Label the electrophilic and...Ch. 3 - Problem 3.27 Considering only electron density,...Ch. 3 - The fact that sweet-tasting carbohydrates like...Ch. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 3.22PCh. 3 - 3.32 Identify the functional groups in each...Ch. 3 - Draw the seven constitutional isomers having...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 3.27PCh. 3 - Prob. 3.28PCh. 3 - Prob. 3.29PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - Prob. 3.32PCh. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 3.34PCh. 3 - Prob. 3.35PCh. 3 - Explain the observed trend in the melting points...Ch. 3 - Prob. 3.37PCh. 3 - Prob. 3.38PCh. 3 - Prob. 3.39PCh. 3 - 3.48 Explain why diethylether and have similar...Ch. 3 - Prob. 3.41PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 3.43PCh. 3 - Prob. 3.44PCh. 3 - THC is the active component in marijuana, and...Ch. 3 - Prob. 3.46PCh. 3 - Prob. 3.47PCh. 3 - Prob. 3.48PCh. 3 - Label the electrophilic and nucleophilic sites in...Ch. 3 - By using only electron density arguments,...Ch. 3 - Prob. 3.51PCh. 3 - Prob. 3.52PCh. 3 - Prob. 3.53PCh. 3 - Prob. 3.54PCh. 3 - Prob. 3.55PCh. 3 - Recall from section 1.10B that there is restricted...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forwarddraw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forward
- Manoharan Mariappan, FR.D., 34) Complete the following reaction starting from hex-1-yne proceeding via different substitution reactions forming 2-heptanone. (25 pts). A Sia₂BH H₂O₂ NaOH Br D Mechanism for reaction D - ether-cleavage: 10 B Ph-MgCI, THF H₁₂O+ D HBr (XS) C TsCl, Py CH3-CH2-CH2-ONaarrow_forwardIn the table below, the correct structure for (2R)-3-methylpentan-2-ol (IUPAC name) can be represented by the letter OH OH HE > ' ÕH C B OH D A/ E OHarrow_forwardPredict the major products of the following organic reaction: + A Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Check Click and drag to start drawing a structure. Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forward
- Why is analysing salt content (using Mohr titration) in both regular & salt reduced tomato sauce important?arrow_forwardIn the image below, correctly name the glassware # _P ( Blank 1) and T ( Blank 2). 景 A W Blank # 1 Blank #2 1000 +19 E E D 0 0-0 G H A A K Π 12 R M N S 0-0-arrow_forwardFeedback: Your answer is incorrect. Predict the major products of the following organic reaction: CN Δ + A ? NC Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. esc Check 80 MH F1 F2 F3 F4 F5 50 @ # C % 95 € Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C A DII F6 F7 F8 7 * 8 Λ & 6 F9 F10 9 0 4arrow_forward
- Incorrect Feedback: Your answer is incorrect. Predict the major products of the following organic reaction: ཤིགས་བྱ རྩ་ཅད་ཀྱིས་༢༩ + Some important notes: A ^ ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. E Check 0 لا Save For La ©2025 McGraw Hill LLC. All Rights Reserved. Terms of All F9 Aarrow_forwardPredict the major products of the following organic reaction: + Δ A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privaarrow_forwardesc 2 Incorrect Feedback: Your answer is incorrect. Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? A O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. . If your answer is no, check the box under the drawing area instead. Check F1 ! @ X C Save For Later Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 80 et A ད 1 4 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 # $ 45 % A 6 87 & * 8 9 ) 0 + ||arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY