
(a)
Interpretation:
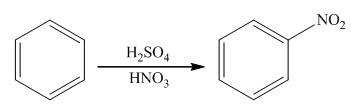
The main product of the mononitration of benzoic acid should be predicted.
Concept introduction:
The electrophilic
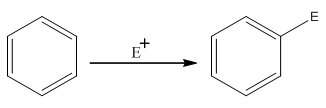
Where,
The activating groups are the groups that have the ability to donate the electron density to the benzene ring.
The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta direction groups are deactivating groups.
The nitration reaction takes place in the presence of nitric acid and sulphuric acid. In this reaction, the protonation of nitric acid occurs in order to produce the nitronium ion. The nitronium ion will attach on the benzene ring to form nitrobenzene. The general reaction is as follows:
(b)
Interpretation:
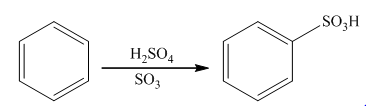
The main product of the monosulphonation of phenol should be predicted.
Concept introduction:
The electrophilic aromatic substitution is the type of reaction in which an electrophile substitutes the hydrogen atom of benzene. A general electrophilic aromatic substitution reaction of benzene can be written as:
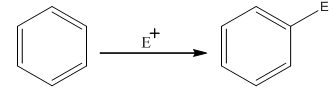
Where,
The activating groups are the groups that have the ability to donate the electron density to the benzene ring.
The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta direction groups are deactivating groups.
The sulfonation takes place in the presence of sulphuric acid. In this reaction, sulfur trioxide is formed that acts as an electrophile. Sulfur trioxide will attach on the benzene ring to form the final product. The general reaction is as follows:
(c)
Interpretation:
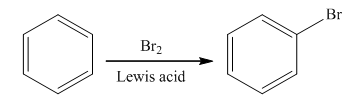
The main product of the monobromination of
Concept introduction:
The electrophilic aromatic substitution is the type of reaction in which an electrophile substitutes the hydrogen atom of benzene. A general electrophilic aromatic substitution reaction of benzene can be written as:
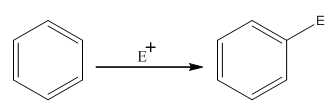
Where,
The activating groups are the groups that have the ability to donate the electron density to the benzene ring.
The deactivating groups are the groups that have the ability to withdraw the electron density to the benzene ring. The ortho and para directing groups are the activating groups while meta direction groups are deactivating groups.
Halogenation is the
The bromination takes place in the presence of Lewis acid and bromine molecule. In this reaction, the bromonium ion is produced that acts as an electrophile. Brominium will attach on the benzene ring to form the final product. The general reaction is as follows:
Trending nowThis is a popular solution!

Chapter 27 Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
- PLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forwardPlease use hand drawn structures when neededarrow_forward
- B. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forwardAnswe Answer A and B pleasearrow_forward
- 3. Refer to the data below to answer the following questions: Isoelectric point Amino Acid Arginine 10.76 Glutamic Acid 3.22 Tryptophan 5.89 A. Define isoelectric point. B. The most basic amino acid is C. The most acidic amino acid is sidizo zoarrow_forward3. A gas mixture contains 50 mol% H2 and 50 mol% He. 1.00-L samples of this gas mixture are mixed with variable volumes of O2 (at 0 °C and 1 atm). A spark is introduced to allow the mixture to undergo complete combustion. The final volume is measured at 0 °C and 1 atm. Which graph best depicts the final volume as a function of the volume of added O2? (A) 2.00 1.75 Final Volume, L 1.50 1.25 1.00 0.75 0.50 0.25 0.00 0.00 0.25 0.50 2.00 (B) 1.75 1.50 Final Volume, L 1.25 1.00 0.75 0.50- 0.25 0.00 0.75 1.00 0.00 0.25 Volume O₂ added, L 2 0.50 0.75 1.00 Volume O₂ added, L 2 2.00 2.00 (C) (D) 1.75 1.75 1.50 1.50 Final Volume, L 1.25 1.00 0.75 0.50 Final Volume, L 1.25 1.00 0.75 0.50 0.25 0.25 0.00 0.00 0.00 0.25 0.50 0.75 1.00 0.00 0.25 Volume O₂ added, L 0.50 0.75 1.00 Volume O₂ added, L 2arrow_forwardLeucine is an essential amino acid with the systematic name 2-amino-3-methylpentanoic acid. It has pai 2.36 and pKa2 = 9.60. H2N-C(R)H-COOH and R is -CH2-CH(CH3)2 A. Draw the condensed structure for leucine, and label all chirality centers with an asterisk. B. How many possible stereoisomers of leucine are there? C. Draw a Fischer projection of L-leucine and label the chirality center(s) as R or S. D. What is the p/ of leucine? E. Draw the structure of the predominant form of leucine at 10.00. F. Draw the structure of the predominant form of leucine at pH = 1.50. G. Leucine is described as an essential amino acid. What does this mean? H. Show the alkyl halide you would use to prepare leucine by the amidomalonate method. =arrow_forward
- a) Write out 6 completely different reactions of acetophenone (reagent, product). b) Write out 3 preparations of 1-methylcyclohexanol, using a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain. c) Write out 3 preparations of 2-ethoxybenzoic acid, a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain.arrow_forward12. CH3 OH OH H&C CH3 H₂C N OH H₂C CH3 H&C CH3 H₂C' CH3 H.C CH3OH H.C CH2CH3OH CH3CEN Which one of these 17 compounds is represented by this IR and this 'H NMR spectrum? IR Spectrum 3000 4000 3000 NMR Spectrum 2000 £500 RAVENUMBER 2000 1500 9 8 6 5 10 HP-00-290 ppm m 1000 500 1000 4 °arrow_forwardDraw the structure of (E,6R) 6-methoxy-4-hepten-2-one. Give the IUPAC name of this compound, including stereochemistry. Draw the most stable chair conformation of (cis) 1,3-isobutylcyclohexane. H HC=CCH₂ CH2CH3 EN(CH3)2 -CN(CH3)2arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

