
Concept explainers
(a)
Interpretation:
The peptide
Concept introduction:
Amino acids are classified as acidic, basic and neutral according to the
Answer to Problem 27.10P
The peptide
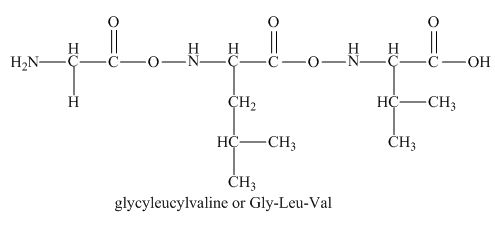
There will be no charge on the peptide,
Explanation of Solution
Peptides are classified as acidic, basic, or neutral according to the amino acids they contain. If they contain unbalanced basic amino acid then they are considered as basic peptide and if they contain unbalanced acidic amino acid then they are considered as basic peptides. If the basic and acidic amino acids are balanced or not at all present in the peptide then it is considered as neutral peptide.
The given peptide

Figure 1
This tripeptide contains one amino group and one carboxylic group. So, this amino acid is neutral in nature. The
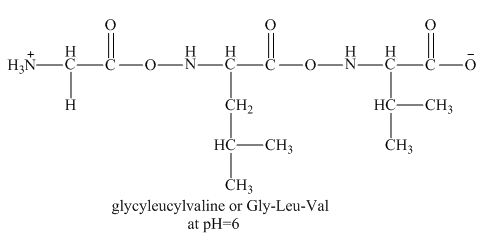
Figure 2
Therefore, the net charge on the peptide,
The peptide
(b)
Interpretation:
The peptide,
Concept introduction:
Amino acids are classified as acidic, basic and neutral according to the functional group they possesses. Acidic amino acids are those which have one more than one carboxylic acid group in its side chain and basic amino acids are those which have one or more amino group present in their side chain. Isoelectric point of the amino acids is the pH of the dilute aqueous solution of the amino acid at which the total charge on all molecules of amino acid is zero.
Answer to Problem 27.10P
The peptide,
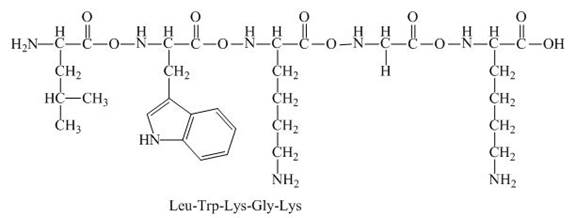
The net charge on the peptide,
Explanation of Solution
Peptides are classified as acidic, basic, or neutral according to the amino acids they contain. If they contain unbalanced basic amino acid then they are considered as basic peptide and if they contain unbalanced acidic amino acid then they are considered as basic peptides. If the basic and acidic amino acids are balanced or not at all present in the peptide then it is considered as neutral peptide.
The given peptide,

Figure 3
This given peptide contains three amino group in its chain and one carboxylic group. So, this amino acid is basic in nature. All the amine group of the given peptide having value of
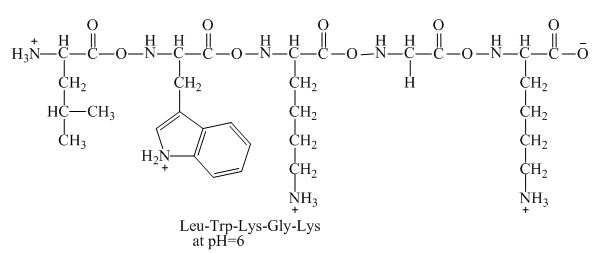
Figure 4
Therefore, the net charge of
The peptide,
(c)
Interpretation:
The peptide,
Concept introduction:
Amino acids are classified as acidic, basic and neutral according to the functional group they possesses. Acidic amino acids are those which have one more than one carboxylic acid group in its side chain and basic amino acids are those which have one or more amino group present in their side chain. Isoelectric point of the amino acids is the pH of the dilute aqueous solution of the amino acid at which the total charge on all molecules of amino acid is zero.
Answer to Problem 27.10P
The peptide
There will be no charge on the peptide,
Explanation of Solution
Peptides are classified as acidic, basic, or neutral according to the amino acids they contain. If they contain unbalanced basic amino acid then they are considered as basic peptide and if they contain unbalanced acidic amino acid then they are considered as basic peptides. If the basic and acidic amino acids are balanced or not at all present in the peptide then it is considered as neutral peptide.
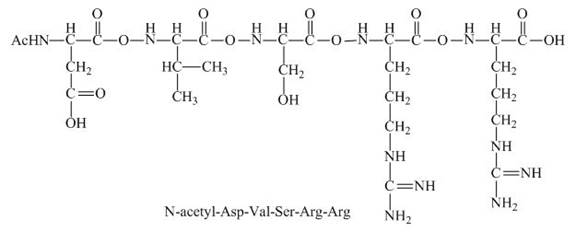
The given peptide

Figure 5
This given peptide contains two amino groups in the side chain and two carboxylic groups. So, this amino acid is neutral in nature. All the amine groups of this peptide having
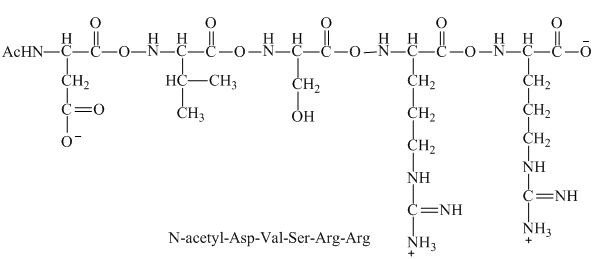
Figure 6
Therefore, the net charge on the peptide,
The peptide
(d)
Interpretation:
The peptide
Concept introduction:
Amino acids are classified as acidic, basic and neutral according to the functional group they possesses. Acidic amino acids are those which have one more than one carboxylic acid group in its side chain and basic amino acids are those which have one or more amino group present in their side chain. Isoelectric point of the amino acids is the pH of the dilute aqueous solution of the amino acid at which the total charge on all molecules of amino acid is zero.
Answer to Problem 27.10P
The peptide
The charge present on the peptide,
Explanation of Solution
Peptides are classified as acidic, basic, or neutral according to the amino acids they contain. If they contain unbalanced basic amino acid then they are considered as basic peptide and if they contain unbalanced acidic amino acid then they are considered as basic peptides. If the basic and acidic amino acids are balanced or not at all present in the peptide then it is considered as neutral peptide.
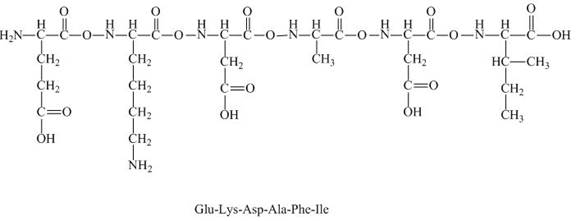
The given peptide

Figure 7
This given peptide contains two amino groups in the side chain and four carboxylic groups. So, this amino acid is acidic in nature. All the amine groups of this peptide having
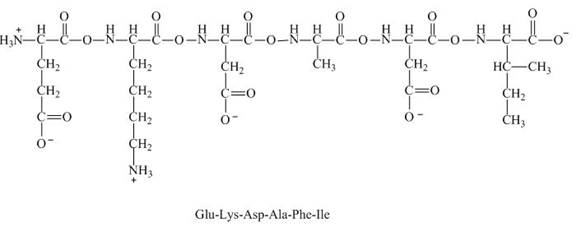
Figure 8
Therefore, the net charge on the peptide,
The peptide
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry
- Select the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forwardSelect the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forward
- Based on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forwardplease help fill in the tablearrow_forwardAnswer F pleasearrow_forward
- 4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward
- . M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forwardPlease use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





