
Concept explainers
Describe what is meant by each of the following reaction types, and illustrate with an example:
(a) nucleophilic substitution reaction: (b) electrophilic substitution reaction; (c) addition reaction;
(d) elimination reaction, (e) rearrangement reaction.
(a)
Interpretation:
The nucleophilic substitution reaction should be defined with example.
Concept introduction:
Nucleophilic substitution reaction describes the attack of the electron-rich group that is nucleophile on electron deficient groups that is electrophile.
Answer to Problem 1E
Nucleophilic substitution reaction is the type of reaction in which the nucleophile (electron rich species) attacks the electron-deficient carbon atom which is electrophilic.
Explanation of Solution
Nucleophilic substitution reaction is defined as an organic reaction which includes the attack of a nucleophile on electrophilic center along with the removal of the leaving group.
The example of the nucleophilic substitution reaction is,
In this reaction, chlorine of chloroethane is replaced by a hydroxyl group
(b)
Interpretation:
The electrophilic substitution reaction should be defined with example.
Concept introduction:
The electrophilic substitution reaction describes the displacement of functional group or hydrogen atom by an electron deficient group or electrophile.
Answer to Problem 1E
Electrophilic substitution reaction is defined as the organic reaction in which the electrophile replaces a functional group of a compound or hydrogen atom.
Explanation of Solution
Electrophilic substitution reaction is defined as the organic reaction which includes the replacement of functional group or
Example of electrophilic substitution reaction is,
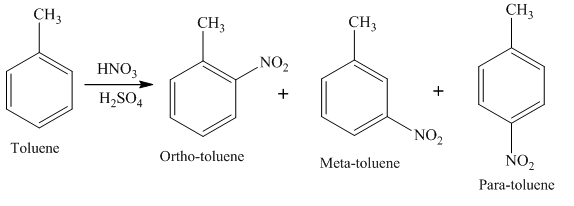
In this reaction, Toluene undergoes electrophilic substitution to form para nitrotoluene, meta nitrotoluene, and ortho nitrotoluene.
(c)
Interpretation:
The addition reaction should be defined with example.
Concept introduction:
The addition reaction describes the combination of two or more smaller molecules to form a larger molecule.
Answer to Problem 1E
Addition reaction is defined as the reaction in which two or more molecules combine to form a single and large molecule.
Explanation of Solution
The reaction of the addition of the two or more reactants that is A and B to produce a single product C is termed as addition reaction.
The example of addition reaction is,
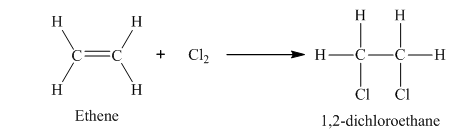
In this reaction, chlorine molecule combines with ethene to form 1, 2-dichloroethane.
(d)
Interpretation:
The elimination reaction should be defined with example.
Concept introduction:
The elimination reaction describes the removal of two substituents from the reactant molecule to form the product.
Answer to Problem 1E
Elimination reaction is the reaction by which the reactant molecule or compound breaks into two or more products.
Explanation of Solution
Elimination reaction is the type of reaction in which two substituents are removed from the reactant molecule to form the product. Generally, unsaturated compounds are formed in an elimination reaction.
The example of the elimination reaction is,
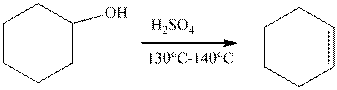
The reaction of cyclohexanol in the presence of
(e)
Interpretation:
The rearrangement reaction should be defined with example.
Concept introduction:
The rearrangement reaction describes the rearrangement of bonds in a molecule to form the product.
Answer to Problem 1E
It is the process of movement of bonds within a molecule to give rise to structural isomers.
Explanation of Solution
It is defined as a reaction in which an atom or a bond migrates from one atom in reactant molecule to adjacent atom to give rise to the product.
Example of rearrangement reaction is,
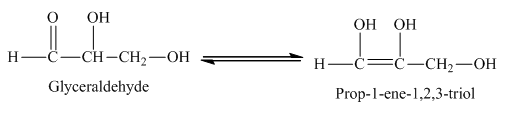
The glyceraldehyde undergoes rearrangement to form enediol.
Want to see more full solutions like this?
Chapter 27 Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
Additional Science Textbook Solutions
Human Physiology: An Integrated Approach (8th Edition)
Organic Chemistry
Physical Universe
Campbell Biology: Concepts & Connections (9th Edition)
General, Organic, and Biological Chemistry - 4th edition
- Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water. If ΔH ° = −56.13 kJ/mol and ΔS ° = 79.11 J/mol ⋅ K, what is the temperature of the reaction if ΔG ° = −80.89 kJ/mol?arrow_forwardFor a particular hypothetical reaction, A+B →2C, the value of AG° is -125 kJ/mol. What is the value of AG for this reaction at 35°C when [A] = 0.10 M, [B] = 0.05 M, and [C] = 2.0 × 10¹ M?arrow_forwardIn an experiment, 74.3 g of metallic copper was heated to 100.0°C and then quickly dropped into 200.0 mL of water in a calorimeter. The heat capacity of the calorimeter with the water was 875 J/°C. The initial temperature of the calorimeter was 27.5°C, and the final temperature after addition of the metal was 29.8°C. What is the value of the molar heat capacity of copper?arrow_forward
- The Haber-Bosch process permits the direct conversion of molecular nitrogen to ammonia, which can be used in large-scale fertilizer production. Given the balanced Haber-Bosch reaction and using the bond energies in the table below, estimate the enthalpy change associated with the reaction. N2(g) + 3H2(g) → 2NH3(g) Bond N=N N = N Energy (kJ/mol) 941 418 N-N H-H N-H 163 435 388arrow_forwardBenzoic acid is used to determine the heat capacity of bomb calorimeters because it can be obtained in pure form and its energy of combustion is known very accurately (−26.43 kJ/g). Determine the heat capacity of a calorimeter that had a temperature increase of 9.199°C when 3.500 g of benzoic acid was used.arrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 2N2H4(g) + 2NO2(g) → 3N2(g) + 4H2O(g) AHrxn ? kJ Substance AH in kJ/mol N2H4(g) +95.4 NO2(g) +33.1 H2O(g) -241.8arrow_forward
- If 7.3 kJ of energy are required to change the temperature of water from 5.0 to 70.0, what was the volume of water? (cs = 4.184 J/(g ⋅ ), d = 1.00 g/mL)arrow_forwardBALANCE CHEMICAL REACTIONarrow_forwardPredict the product(s) of the following reactions. If no reaction, write "NR". a) Cl₂ FeCl3 e) HNO3 H2SO4 b) NO2 CI. HNO3 f) Br Br2 OH H2SO4 HO3S. FeBr3 c) Cl2 g) FeCl3 F d) O₂N Br2 FeBr3 O₂N OH HNO3 CH3 H2SO4arrow_forward
- ulating the pH salt solution Calculate the pH at 25 °C of a 0.75M solution of anilinium chloride (C6H5NH3C1). Note that aniline (C6H5NH2) is a weak base with a pK of 4.87. Round your answer to 1 decimal place. pH = ☐ ☑ ⑤ ? olo 18 Ararrow_forwardI apologize, but the app is not allowing me to post the other 4 pictures of the thermodynamics chart. But I believe the values are universal. Please help!arrow_forwardCalculating the pH of a salt solution Calculate the pH at 25 °C of a 0.29M solution of potassium butanoate (KC3H,CO2). Note that butanoic acid (HC3H,CO2) is a weak acid with a pKa of 4.82. Round your answer to 1 decimal place. pH = -0 Х olo 18 Ararrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





