
(a)
Interpretation: The primary, secondary or tertiary amine in the given compound is to be classified.
Concept introduction:
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 25.1P
Each amine in the given compound is classified as,
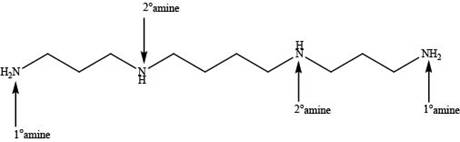
Explanation of Solution
The given compound is shown below.
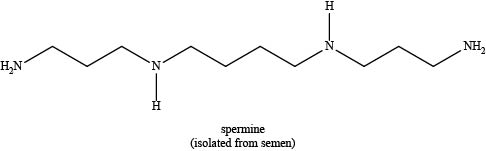
Figure 1
In spermine, four nitrogen atoms are present. Among these four nitrogen atoms, two are primary amines that contain one alkyl group on nitrogen atom and two are secondary amines that contain two alkyl groups on nitrogen atom as shown below.
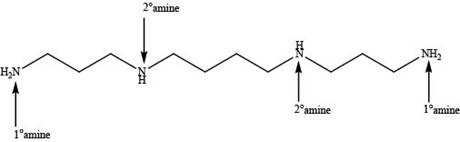
Figure 2
In spermine, primary and secondary amines are present as shown in Figure 2.
(b)
Interpretation: The primary, secondary or tertiary amine in the given compound is to be classified.
Concept introduction: Amines are nitrogen-containing organic compounds. The general formula of amines is
In secondary amines, two of the hydrogen atoms of ammonia are replaced by alkyl or aryl group.
In tertiary amines, all hydrogen atoms of ammonia are replaced by alkyl or aryl group.
Answer to Problem 25.1P
Each amine in the given compound is classified as,
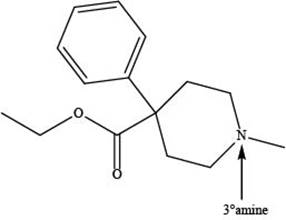
Explanation of Solution
The given compound is shown below.
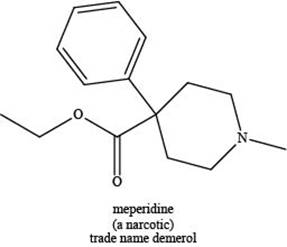
Figure 3
Meperidine contains one nitrogen atom bonded to three alkyl groups. Hence, it is a tertiary amine as shown below.

Figure 4
In meperidine, tertiary amine is present as shown in Figure 4.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- Check the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. CH3 CH2 0 C=O + CH-CH3 H₂N C-COOH H₂N H H H3N C COO¯ NH, O HO C C H CH3-CH HO C=O H2N-CH-COOH CH2 NH3 HO CH3 none of them O NH3arrow_forwardhandwritten answer please!arrow_forwardConsider the following SN 2 reaction: مار + Br H₂O acetone + Br OH What effect would each of the following changes have on the rate of this reaction. Select the single best answer for each part. Part 1 of 3 If the substrate was changed to: The rate would Br O increase O decrease O remain unchanged Part 2 of 3 × S If the nucleophile was changed to OH, the rate would: O increase O decrease O remain unchanged Part 3 of 3 If the solvent was changed to ethanol, the rate would: Increase O decrease O remain unchanged 2 ol Ararrow_forward
- Consider the following nucleophilic substitution reaction. The compound listed above the arrow is the solvent for the reaction. If nothing is listed over the arrow, then the nucleophile is also the solvent for the reaction. Part: 0/2 Part 1 of 2 Br acetone + I What is the correct mechanism for the reaction? Select the single best answer. OSN 1 OSN 2 X Part: 1/2 Part 2 of 2 Draw the products for the reaction. Include both the major organic product and the inorganic product. If more than one stereoisomer is possible, draw only one stereoisomer. Include stereochemistry where relevant. Click and drag to start drawing a structure. Х 5 ☐arrow_forwardTriethyloxonium tetrafluoroborate reacts with ethanol (CH3CH2OH) to give diethyl ether (CH3CH2OCH2CH3). BF triethyloxonium tetrafluoroborate Which equation, including the curved arrows, best represents the rate-determining step in the mechanism? Select the single best answer. O OH CH3CH2 OH + H. 0+ CH₂H₂ :0 + 0+ ж + H + :0: 0 Carrow_forwardCH3CH2CH=CH2 + H₂O − H+arrow_forward
- Г C-RSA CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0.0 b.092 0.797 1.088 1.813 C-RSA CHROMATOPAC CH=1 Report No. =13 ** CALCULATION REPORT ** DATA=1: @CHRM1.000 11/03/05 08:09:52 CH PKNO TIME 1 2 0.797 3 1.088 4 1.813 AREA 1508566 4625442 2180060 HEIGHT 207739 701206 V 287554 V MK IDNO CONC NAME 18.1447 55.6339 26.2213 TOTAL 8314067 1196500 100 C-R8A CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0. 0 087 337. 0.841 1.150 C-R8A CHROMATOPAC CH=1 Report No. =14 DATA=1: @CHRM1.000 11/03/05 08:12:40 ** CALCULATION REPORT ** CH PKNO TIME AREA 1 3 0.841 1099933 41.15 4039778 HEIGHT MK IDNO 170372 649997¯¯¯ CONC NAME 21.4007 78.5993 TOTAL 5139711 820369 100 3 C-R8A CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0.100 0:652 5.856 3 1.165 C-RSA CHROMATOPAC CH-1 Report No. =15 DATA=1: @CHRM1.000 11/03/05 08:15:26 ** CALCULATION REPORT ** CH PKNO TIME AREA HEIGHT MK IDNO CONC NAME 1 3 3 0.856 4 1.165 TOTAL 1253386 4838738 175481 708024 V 20.5739 79.4261 6092124…arrow_forwardIndicate the product that is obtained if the benzotriazole reacts with the use of a medium basic product.arrow_forwardIndicate the product that is obtained if the benzotriazol reacts with dimethyl sulfate.arrow_forward
- Indicate how to obtain 2-metilbencimidazol from 1,2-diaminobenzene.arrow_forwardbreak down both reactions shown and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanism.arrow_forwardIndicate how from 1,2-diaminobenzene to obtain 1-metilbenzotriazol.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning




