
Synthesize each compound from benzene. Use a diazonium salt as one of the synthetic intermediates.
a. 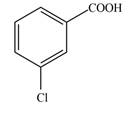 c.
c. 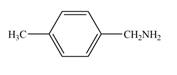 e.
e.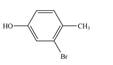
b. 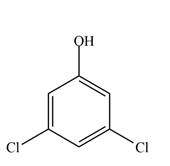 d.
d. 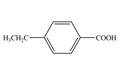 f.
f.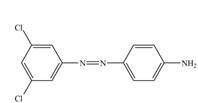
(a)
Interpretation: The given compound is to be synthesized from benzene by the use of diazonium salt as one of the synthetic intermediates.
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.80P
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
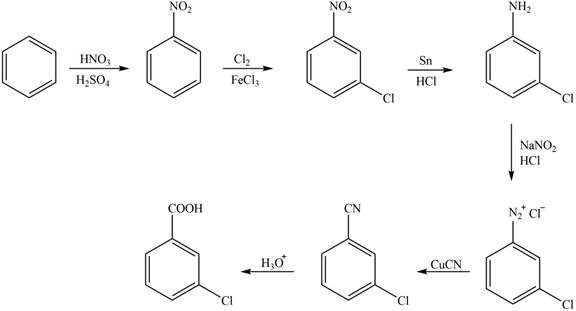
Explanation of Solution
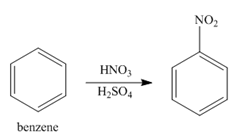
In the first step of the synthesis, nitration of benzene ring takes place. The nitro group is meta directing, as a result the chlorination takes place at meta position in the second step. The nitro group converts to amine group on reaction with
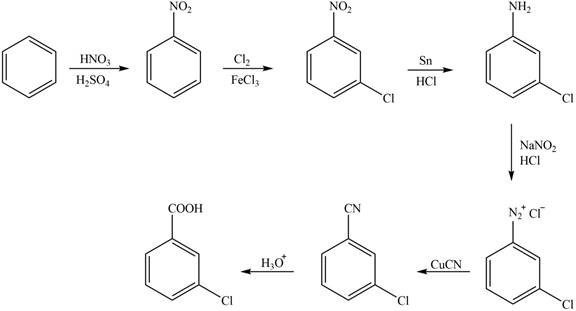
Figure 1
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown in Figure 1.
(b)
Interpretation: The given compound is to be synthesized from benzene by the use of diazonium salt as one of the synthetic intermediates.
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.80P
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
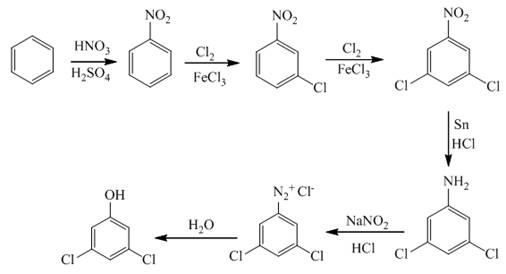
Explanation of Solution
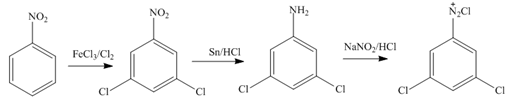
In the first step of the synthesis, nitration of benzene ring takes place. The nitro group is meta directing, as a result the chlorination takes place at meta position in the next two steps. The nitro group converts to amine group on reaction with
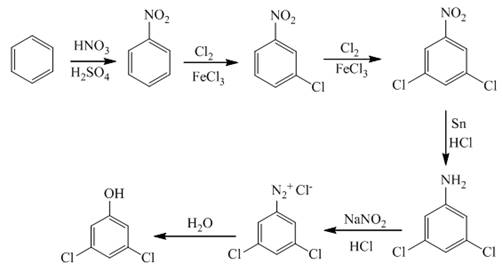
Figure 2
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown in Figure 2.
(c)
Interpretation: The given compound is to be synthesized from benzene by the use of diazonium salt as one of the synthetic intermediates.
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.80P
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
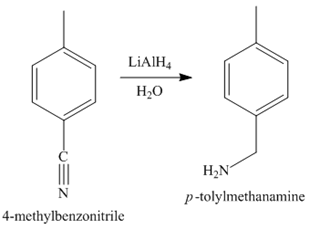
Explanation of Solution
The first step of synthesis of given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
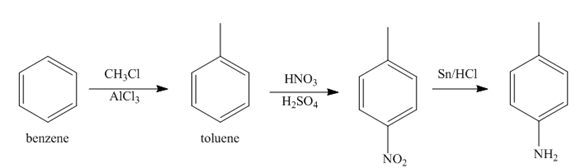
Figure 3
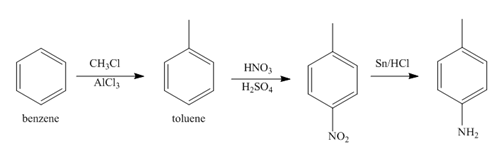
In the above step, benzene undergoes Friedel craft’s alkylation reaction to form toluene. It further reacts with
The second step of synthesis of given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
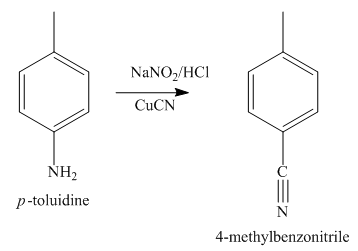
Figure 4
The compound
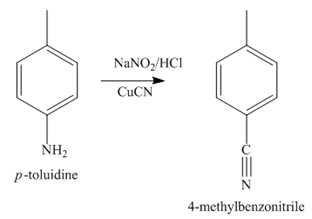
The third step of synthesis of given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
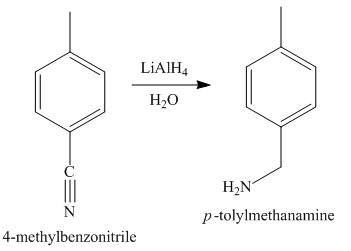
Figure 5
In the last step, the reduction of nitrile to amine takes place in the presence of
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown in Figure 3, Figure 4 and Figure 5.
(d)
Interpretation: The given compound is to be synthesized from benzene by the use of diazonium salt as one of the synthetic intermediates.
Concept introduction: Amines are the derivatives of ammonia consisting of a nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.80P
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
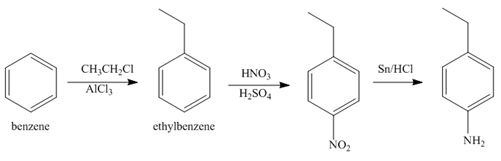
Explanation of Solution
The first step of synthesis of given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
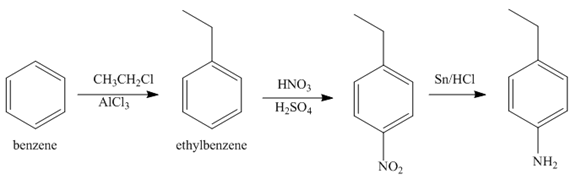
Figure 6
In the above step, benzene undergoes Friedel craft’s alkylation reaction to form ethylbenzene. It further reacts with
The second step of synthesis of given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
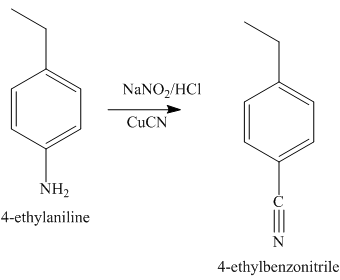
Figure 7
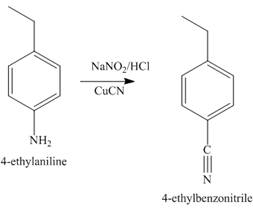
The compound
The third step of synthesis of given compound from benzene, by the use of a diazonium salt as one of the synthetic intermediates, is shown below.
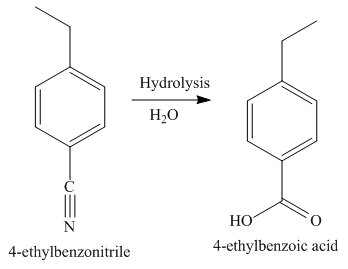
Figure 8
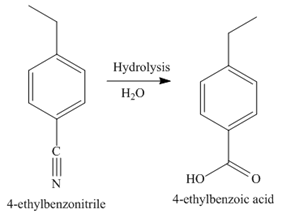
In the last step, the hydrolysis reaction takes place, which results in the formation of
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown in Figure 6, Figure 7 and Figure 8.
(e)
Interpretation: The given compound is to be synthesized from benzene by the use of diazonium salt as one of the synthetic intermediates.
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.80P
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
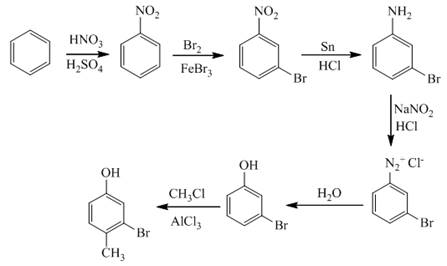
Explanation of Solution
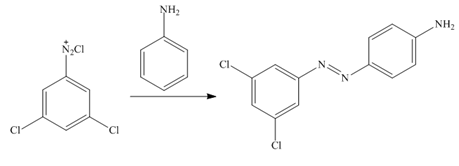
In the first step of the synthesis, nitration of benzene ring takes place. The nitro group is meta directing, as a result the bromination takes place at meta position in the second step. The nitro group converts to amine group on reaction with
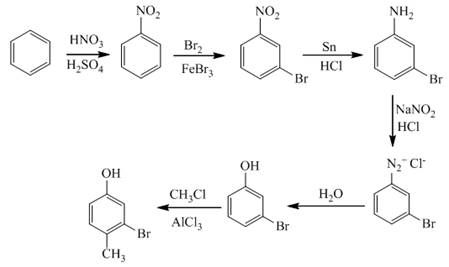
Figure 9
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown in Figure 9.
(f)
Interpretation: The given compound is to be synthesized from benzene by the use of diazonium salt as one of the synthetic intermediates.
Concept introduction: Amines are the derivatives of ammonia consisting of a nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.80P
The synthesis of given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates is shown below.
Explanation of Solution
The first step of synthesis of given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
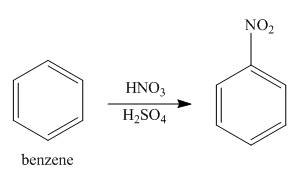
Figure 10
In the above step, benzene reacts with
The second step of synthesis of given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
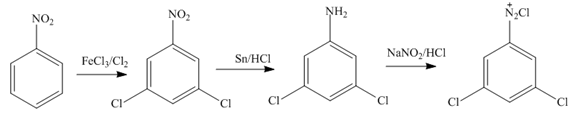
Figure 11
Nitrobenzene undergoes chlorination and reduction reaction to form
The third step of synthesis of given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown below.
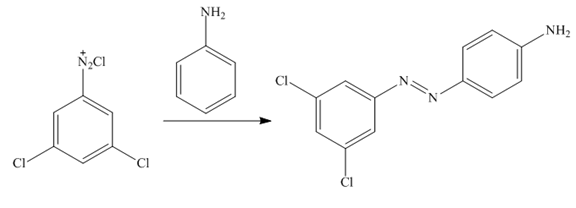
Figure 12
In the last step,
The synthesis of a given compound from benzene, by the use of diazonium salt as one of the synthetic intermediates, is shown in Figure 10, Figure 11 and Figure 12.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

