
Draw the products formed when
a.
b.
c.
d. excess
e.
f.
g.
h.
i. Part (b), then
j.
(a)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
The product formed by the treatment of
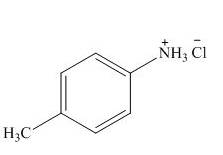
Explanation of Solution
The product formed by the treatment of
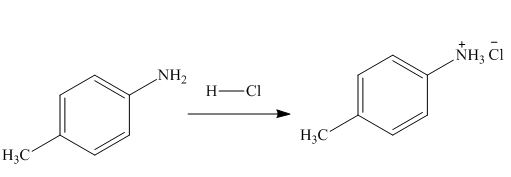
Figure 1
In the above reaction, hydrochloric acid attacks on nitrogen atom of
The product formed by the treatment of
(b)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
The products formed by the treatment of
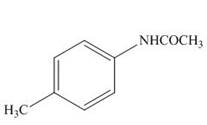
Explanation of Solution
The products formed by the treatment of
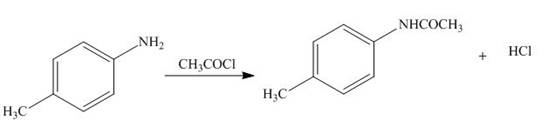
Figure 2
The above reaction indicates that
The products formed by the treatment of
(c)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
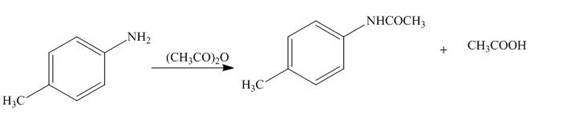
Figure 3
The above reaction implies that
The products formed by the treatment of
(d)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
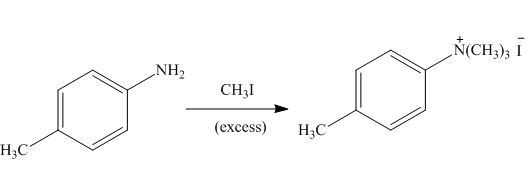
Figure 4
The above reaction indicates that excess of methyl iodide reacts with
The products formed by the treatment of
(e)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
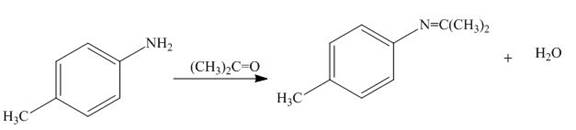
Figure 5
Water gets removed when
The products formed by the treatment of
(f)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
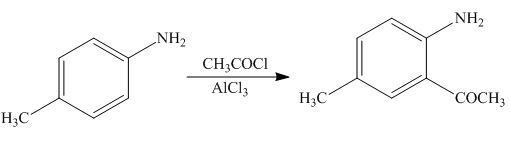
Figure 6
The above reaction indicates that
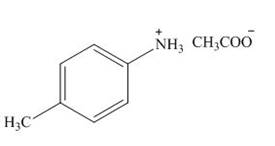
(g)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
The product formed by the treatment of
Explanation of Solution
The product formed by the treatment of
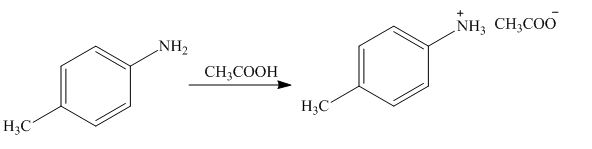
Figure 7
The above reaction indicates that
The product formed by the treatment of
(h)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
The product formed by the treatment of
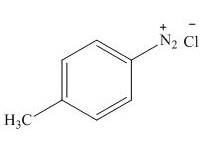
Explanation of Solution
The product formed by the treatment of
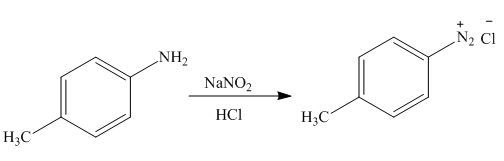
Figure 8
In the above reaction
The product formed by the treatment of
(i)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
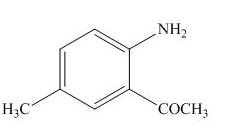
The product formed by the treatment of
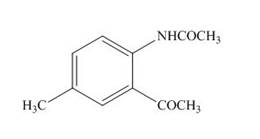
Explanation of Solution
The product formed by the treatment of
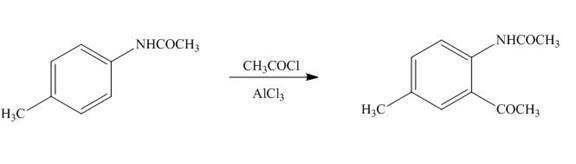
Figure 9
The product formed in part (b) is used as a reactant in the above reaction. It again reacts with
The product formed by the treatment of
(j)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.60P
The product formed by the treatment of
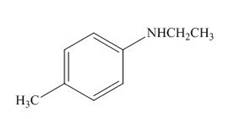
Explanation of Solution
The product formed by the treatment of
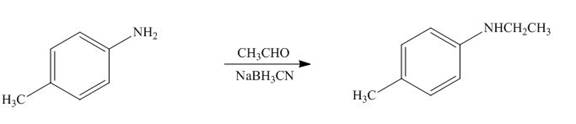
Figure 10
In the above reaction,
The product formed by the treatment of
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- What is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forward
- Please use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forward
- Answe Answer A and B pleasearrow_forward3. Refer to the data below to answer the following questions: Isoelectric point Amino Acid Arginine 10.76 Glutamic Acid 3.22 Tryptophan 5.89 A. Define isoelectric point. B. The most basic amino acid is C. The most acidic amino acid is sidizo zoarrow_forward3. A gas mixture contains 50 mol% H2 and 50 mol% He. 1.00-L samples of this gas mixture are mixed with variable volumes of O2 (at 0 °C and 1 atm). A spark is introduced to allow the mixture to undergo complete combustion. The final volume is measured at 0 °C and 1 atm. Which graph best depicts the final volume as a function of the volume of added O2? (A) 2.00 1.75 Final Volume, L 1.50 1.25 1.00 0.75 0.50 0.25 0.00 0.00 0.25 0.50 2.00 (B) 1.75 1.50 Final Volume, L 1.25 1.00 0.75 0.50- 0.25 0.00 0.75 1.00 0.00 0.25 Volume O₂ added, L 2 0.50 0.75 1.00 Volume O₂ added, L 2 2.00 2.00 (C) (D) 1.75 1.75 1.50 1.50 Final Volume, L 1.25 1.00 0.75 0.50 Final Volume, L 1.25 1.00 0.75 0.50 0.25 0.25 0.00 0.00 0.00 0.25 0.50 0.75 1.00 0.00 0.25 Volume O₂ added, L 0.50 0.75 1.00 Volume O₂ added, L 2arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning



