
Concept explainers
(a)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagent that is required to convert acetophenone
Explanation of Solution
The given compound is shown below.
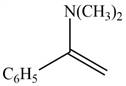
Figure 1
Carbonyl compounds react with secondary
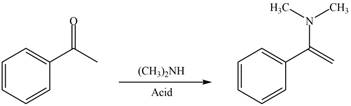
Figure 2
Thus, the reagent that is required to convert acetophenone
The reagent that is required to convert acetophenone
(b)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagent that is required to convert acetophenone
Explanation of Solution
The given compound is shown below.
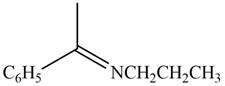
Figure 3
Carbonyl compounds react with primary amines in the presence of mild acid to form imine. Therefore, the reaction that shows the conversion of acetophenone into the given compound is shown below.
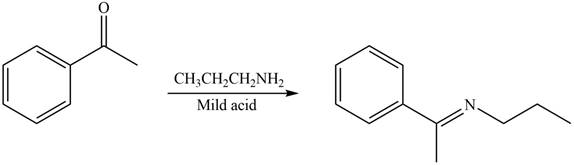
Figure 4
Thus, the reagent that is required to convert acetophenone
The reagent that is required to convert acetophenone
(c)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
he reagents that are required to convert acetophenone
Explanation of Solution
The given compound is shown below.
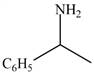
Figure 5
Carbonyl compounds react with ammonia to form imine. The conversion of imine to amines takes place by using selective reducing agent called sodium cyanoborohydride. Therefore, the reaction that shows the conversion of acetophenone into the given compound is shown below.
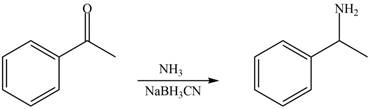
Figure 6
Thus, reagent that is required to convert acetophenone
The reagents that are required to convert acetophenone
(d)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagents that are required to convert acetophenone
Explanation of Solution
The given compound is shown below.
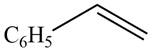
Figure 7
The reaction that shows the conversion of acetophenone into the given compound is shown below.
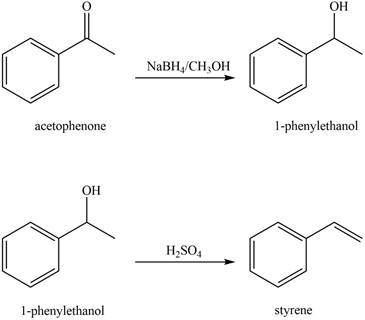
Figure 8
The given reaction is a two step process. The first step involves reduction of carbonyl compounds into alcohol by using sodium borohydride. This results in the formation of
Thus, the reagents that are required to convert acetophenone
The reagents that are required to convert acetophenone
(e)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagents that are required to convert acetophenone
Explanation of Solution
The given compound is shown below.
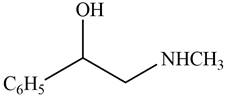
Figure 9
The reaction that shows the conversion of acetophenone into the given compound is shown below.
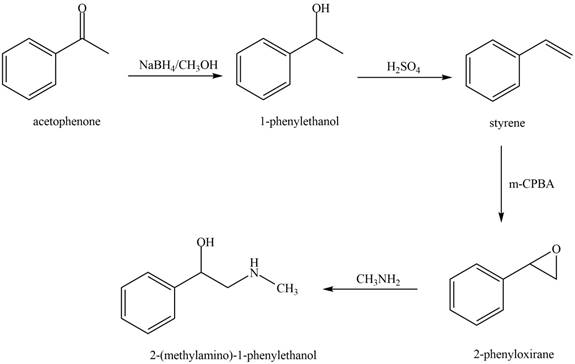
Figure 10
The first step involves reduction of carbonyl compounds into alcohol by using sodium borohydride. This results in the formation of
Thus, the reagents that are required to convert acetophenone
The reagents that are required to convert acetophenone
(f)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagent that is required to convert acetophenone
Explanation of Solution
The given compound is shown below.
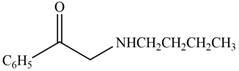
Figure 11
The reaction that shows the conversion of acetophenone into the given compound is shown below.
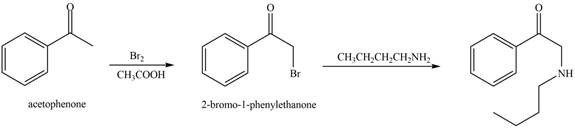
Figure 12
The first step is the bromination step which results in the formation of
Thus, reagent that is required to convert acetophenone
The reagent that is required to convert acetophenone
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- For each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forwardPredict the major products of this organic reaction: OH NaBH4 H ? CH3OH Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. ☐ : Sarrow_forwardPredict the major products of this organic reaction: 1. LIAIHA 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. X : ☐arrow_forward
- For each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forward
- give example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward
- 3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forwardWhat is the reason of the following(use equations if possible) a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5 d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt e.) Cbvcarrow_forwardA 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72. Determine the molar mass of the acid. Determine the Ka of the acid.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





