
Concept explainers
For alanine,
(a) 2.00.
(b) 6.00.
(c) 10.50.
What is the principal species at each pH?
(a)
Interpretation:
The value of
Concept introduction:
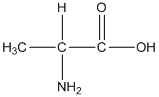
General formula of alanine is shown as follows:
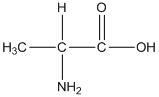
The
Answer to Problem 29QAP
The principal species at
Explanation of Solution
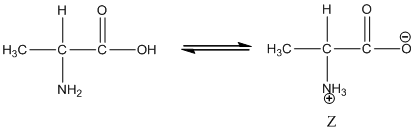
Under equilibrium condition, the Zwitter ionic form of alanine is shown as follows:
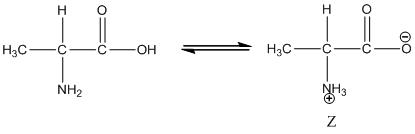
Under acidic condition, oxygen atom accepts the proton Zwitter ion exists in cationic form.
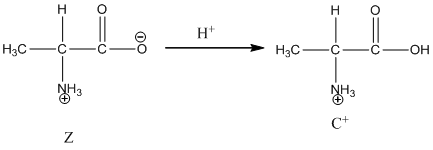
Under basic condition, base abstracts proton form nitrogen atom and it forms the anionic form.
Dissociation of cation is represented as follows:
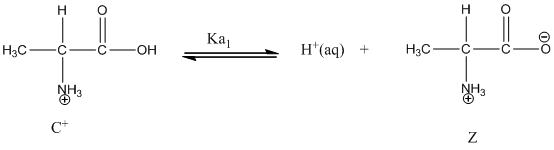
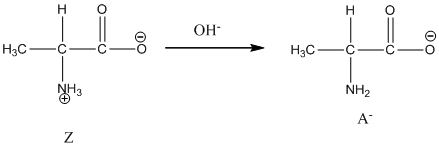
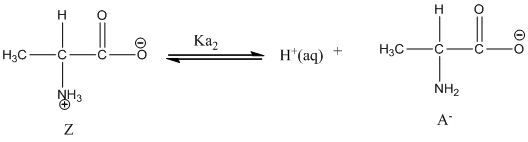
Dissociation of anion is represented as follows:
Given value of
At
So,
Take antilog both sides,
Here, Z is Zwitter ion and C is cationic form of Zwitter ion.
Here, Z is Zwitter ion and A is anionic form of Zwitter ion.
Therefore,
The principal species at
(b)
Interpretation:
The value of
Concept introduction:
General formula of alanine is shown as follows:
The
Answer to Problem 29QAP
The principal species at
Explanation of Solution
Under equilibrium condition zwitter ionic form of alanine is:
Under acidic condition oxygen atom accepts the proton zwitter ion exists in cationic form.
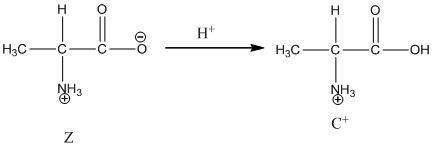
Under basic condition base abstracts proton form nitrogen atom and it forms the anionic form.
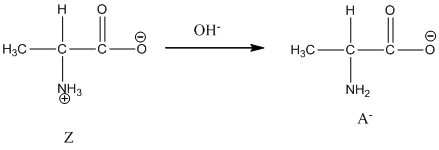
Dissociation of cation is-
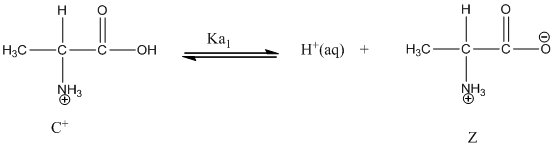
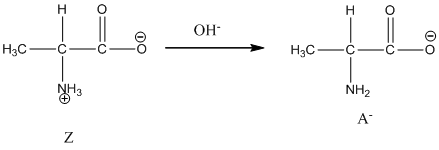
Dissociation of anion is-
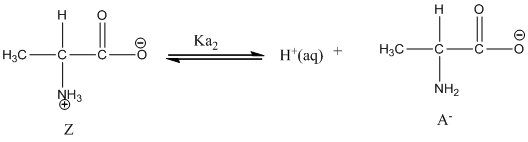
Given value of
At
So,
Take antilog both sides,
Here, Z is zwitter ion and C is cationic form of zwitter ion.
Here, Z is zwitter ion and A is anionic form of zwitter ion.
The principal species at
(c)
Interpretation:
The value of
Concept introduction:
General formula of alanine is shown as follows:
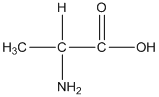
The
Answer to Problem 29QAP
The principal species at
Explanation of Solution
Under equilibrium condition zwitter ionic form of alanine is:
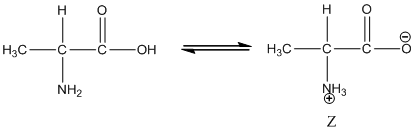
Under acidic condition oxygen atom accepts the proton zwitter ion exists in cationic form.
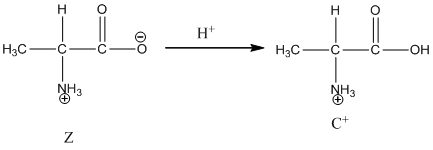
Under basic condition base abstracts proton form nitrogen atom and it forms the anionic form.
Dissociation of cation is-
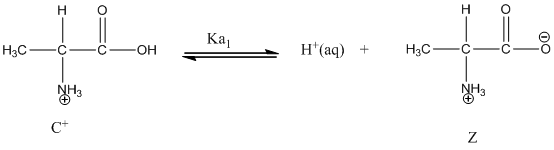
Dissociation of anion is-
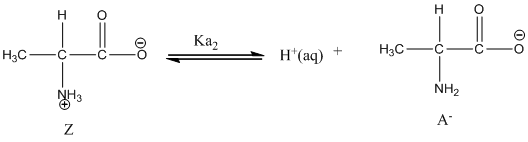
Given value of
At
So,
Take antilog both sides,
Here, Z is zwitter ion and C is cationic form of zwitter ion.
Here, Z is zwitter ion and A is anionic form of zwitter ion.
The principal species at
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: Principles and Reactions
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
- Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





