
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 13P
How would you transform tetradecanal into each of the following?
(a)
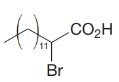
(b)
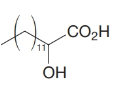
(c)
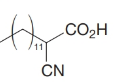
(d)
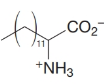
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Help fix my arrows please
no Ai walkthroughs
Given the data attached, provide a drawing of the corresponding structure.
Chapter 23 Solutions
Organic Chemistry
Ch. 23 - Prob. 1PPCh. 23 - Prob. 2PPCh. 23 - Prob. 3PPCh. 23 - Prob. 4PPCh. 23 - Prob. 5PPCh. 23 - Prob. 6PPCh. 23 - Prob. 7PPCh. 23 - Prob. 8PPCh. 23 - Prob. 9PPCh. 23 - Prob. 10PP
Ch. 23 - Prob. 11PPCh. 23 - Prob. 12PCh. 23 - 23.15 How would you transform tetradecanal into...Ch. 23 - Prob. 14PCh. 23 - Prob. 15PCh. 23 - When limonene (Section 23.3) is heated strongly,...Ch. 23 - Gadoleic acid (C20H38O2), a fatty acid that can be...Ch. 23 - 23.20 -Phellandrene and -phellandrene are isomeric...Ch. 23 - Prob. 19PCh. 23 - Prob. 20PCh. 23 - Prob. 21PCh. 23 - The initial steps of a laboratory synthesis of...Ch. 23 - Prob. 23PCh. 23 - Prob. 24PCh. 23 - Prob. 25PCh. 23 - 2. The biosynthesis of fatty acids is accomplished...Ch. 23 - Prob. 3LGPCh. 23 - Prob. 4LGPCh. 23 - 23.1 Write an appropriate formula in each box.
Ch. 23 - Give a reagent that would distinguish between each...Ch. 23 - 23.3 What product would be obtained by catalytic...Ch. 23 - Prob. 4QCh. 23 - 23.5 The following compound is a:
(a)...Ch. 23 - Mark off the isoprene units in the previous...Ch. 23 - Prob. 7Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
1.2 Ask two of your friends (not in class) to define the terms in problem1.1.
Do their answers agee with the d...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
What type of cut would separate the brain into anterior and posterior parts?
Anatomy & Physiology (6th Edition)
The reason of expressing heat of combustion in negative value needs to be described. Concept introduction: Comb...
Living By Chemistry: First Edition Textbook
Where are skeletal cartilages located?
Human Anatomy & Physiology (2nd Edition)
How much more water vapor is contained in 1 kilogram of saturated air at 35C than in 1 kilogram of saturated ai...
Applications and Investigations in Earth Science (9th Edition)
Define isotopes and free radicals.
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
- 3) Draw a detailed mechanism and predict the product of the reaction shown? 1) EtMgBr 2) H3O+arrow_forwardHow to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License