
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
4th Edition
ISBN: 9781265982959
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.2, Problem 21.2P
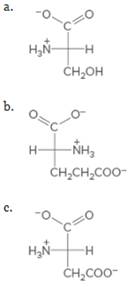
Which of the following amino acids is naturally occurring? By referring to the structures in Table 21.2, name each amino acid and include its D or L designation in the name.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!
Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!
Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!
Chapter 21 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
Ch. 21.2 - In addition to the amino and carboxyl groups, what...Ch. 21.2 - Draw both enantiomers of each amino acid in...Ch. 21.2 - Which of the following amino acids is naturally...Ch. 21.3 - Draw the structure of the amino acid valine at...Ch. 21.3 - Identify the amino acid shown with all uncharged...Ch. 21.4 - Identify the N-terminal and C-terminal amino acid...Ch. 21.4 - Prob. 21.4PCh. 21.4 - Prob. 21.4PPCh. 21.4 - Prob. 21.5PCh. 21.4 - Prob. 21.5PP
Ch. 21.4 - Prob. 21.6PPCh. 21.5 - Prob. 21.6PCh. 21.6 - Prob. 21.7PCh. 21.6 - Prob. 21.8PCh. 21.6 - Prob. 21.9PCh. 21.7 - Why is hemoglobin more water soluble than ...Ch. 21.8 - Prob. 21.7PPCh. 21.8 - Prob. 21.11PCh. 21.9 - Prob. 21.8PPCh. 21.9 - Prob. 21.12PCh. 21.9 - Prob. 21.9PPCh. 21.9 - Prob. 21.13PCh. 21.10 - Prob. 21.14PCh. 21.10 - Prob. 21.15PCh. 21.10 - Prob. 21.16PCh. 21.10 - Prob. 21.17PCh. 21.10 - The nerve gas sarin acts as a poison by covalently...Ch. 21.10 - Prob. 21.19PCh. 21.10 - Explain why the proteins involved in blood...Ch. 21 - The amino acid alanine is a solid at room...Ch. 21 - Why is phenylalanine water soluble but...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - Draw both enantiomers of each amino acid and label...Ch. 21 - Which of the following Fischer projections...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - (a) Identify the amino acid shown with all...Ch. 21 - Prob. 32PCh. 21 - Prob. 33PCh. 21 - Draw the structure of the neutral, positively...Ch. 21 - Locate the peptide bond in the dipeptide shown in...Ch. 21 - Label the N-terminal and C-terminal amino acids in...Ch. 21 - Melittin, the principal toxin of bee venom,...Ch. 21 - Cobratoxin is a neurotoxin found in the venom of...Ch. 21 - (a) Draw the structure of the two possible...Ch. 21 - (a) Draw the structure of the two possible...Ch. 21 - For each tripeptide: [1] draw the structure of the...Ch. 21 - For each tripeptide: [1] draw the structure of the...Ch. 21 - Prob. 43PCh. 21 - For each tripeptide: [1] identify the amino acids...Ch. 21 - What amino acids are formed by hydrolysis of the...Ch. 21 - Prob. 46PCh. 21 - Prob. 47PCh. 21 - Draw the structures of the amino acids formed when...Ch. 21 - Prob. 49PCh. 21 - Prob. 50PCh. 21 - Prob. 51PCh. 21 - Trypsin is a digestive enzyme that hydrolyzes...Ch. 21 - What type of intermolecular forces exist between...Ch. 21 - What type of interaction occur at each of the...Ch. 21 - Which peptide in each pair contains amino acids...Ch. 21 - Decide if the side chains of the amino acid...Ch. 21 - Which type of protein structure is indicated in...Ch. 21 - Label each of the following diagrams as...Ch. 21 - Prob. 59PCh. 21 - Prob. 60PCh. 21 - Prob. 61PCh. 21 - Prob. 62PCh. 21 - Compare - keratin and hemoglobin with regard to...Ch. 21 - Compare collagen and myoglobin with regard to each...Ch. 21 - Prob. 65PCh. 21 - Prob. 66PCh. 21 - Describe the function or biological activity of...Ch. 21 - Describe the function or biological activity of...Ch. 21 - Prob. 69PCh. 21 - Prob. 70PCh. 21 - What class of enzyme catalyzes each of the...Ch. 21 - What class of enzyme catalyzes each of the...Ch. 21 - Prob. 73PCh. 21 - Prob. 74PCh. 21 - Prob. 75PCh. 21 - What kind of reaction is catalyzed by each of the...Ch. 21 - Prob. 77PCh. 21 - How will each of the following changes affect the...Ch. 21 - Prob. 79PCh. 21 - Prob. 80PCh. 21 - Prob. 81PCh. 21 - Prob. 82PCh. 21 - Prob. 83PCh. 21 - Prob. 84PCh. 21 - Prob. 85PCh. 21 - Prob. 86PCh. 21 - Why must vegetarian diets be carefully balanced?Ch. 21 - Prob. 88PCh. 21 - Sometimes an incision is cauterized (burned) to...Ch. 21 - Why is insulin administered by injection instead...Ch. 21 - Prob. 91PCh. 21 - The silk produced by a silkworm is a protein with...Ch. 21 - Explain the difference in the mechanism of action...Ch. 21 - Prob. 94PCh. 21 - Prob. 95CPCh. 21 - Suggest a reason for the following observation....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer the questions in the photos and please revise any wrong answers. Thank youarrow_forward(Please be sure that 7 carbons are available in the structure )Based on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10.arrow_forward-lease help me answer the questions in the photo.arrow_forward
- For the reaction below, the concentrations at equilibrium are [SO₂] = 0.50 M, [0] = 0.45 M, and [SO3] = 1.7 M. What is the value of the equilibrium constant, K? 2SO2(g) + O2(g) 2SO3(g) Report your answer using two significant figures. Provide your answer below:arrow_forwardI need help with this question. Step by step solution, please!arrow_forwardZn(OH)2(s) Zn(OH)+ Ksp = 3 X 10-16 B₁ = 1 x 104 Zn(OH)2(aq) B₂ = 2 x 1010 Zn(OH)3 ẞ3-8 x 1013 Zn(OH) B4-3 x 1015arrow_forward
- Help me understand this by showing step by step solution.arrow_forwardscratch paper, and the integrated rate table provided in class. our scratch work for this test. Content attribution 3/40 FEEDBACK QUESTION 3 - 4 POINTS Complete the equation that relates the rate of consumption of H+ and the rate of formation of Br2 for the given reaction. 5Br (aq) + BrO3 (aq) + 6H (aq) →3Br2(aq) + 3H2O(l) • Your answers should be whole numbers or fractions without any decimal places. Provide your answer below: Search 尚 5 fn 40 * 00 99+ 2 9 144 a [arrow_forward(a) Write down the structure of EDTA molecule and show the complex structure with Pb2+ . (b) When do you need to perform back titration? (c) Ni2+ can be analyzed by a back titration using standard Zn2+ at pH 5.5 with xylenol orange indicator. A solution containing 25.00 mL of Ni2+ in dilute HCl is treated with 25.00 mL of 0.05283 M Na2EDTA. The solution is neutralized with NaOH, and the pH is adjusted to 5.5 with acetate buffer. The solution turns yellow when a few drops of indicator are added. Titration with 0.02299 M Zn2+ requires 17.61 mL to reach the red end point. What is the molarity of Ni2+ in the unknown?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY