
Concept explainers
(a)
Interpretation:
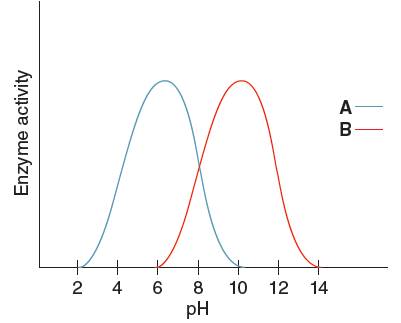
The optimum pH for enzyme 'A' needs to be determined, using the given pH versus enzyme activity graph for enzyme A and B.
Concept Introduction:
Enzymes are defined as a biological catalyst for various reactions in the living organisms. Enzymes are like all catalyst as it also increases the rate reaction but they themselves do not changed permanently during the process. Enzyme does not alter the position of equilibrium and the relative energy of both the reactant and the products. Reaction when catalyzed by an enzyme increases the
Answer to Problem 77P
Optimum pH for enzyme 'A' is
Explanation of Solution
The activity of enzyme is affected by both the temperature and pH. By increasing temperature, rate of reaction also increases until its maximum activity occurs that is at
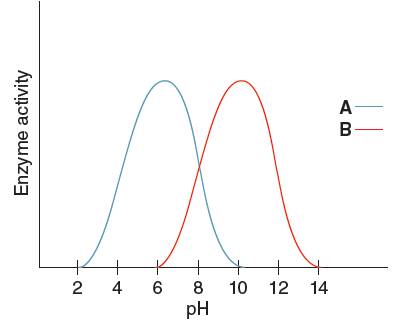
Optimum pH is the pH at which the activity of an enzyme is maximum. So, from the given graph, the activity of enzyme A is maximum at pH 6. Hence the optimum pH of enzyme A is 6.
(b)
Interpretation:
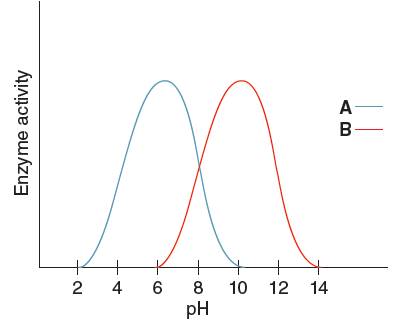
The optimum pH for enzyme 'B' needs to be determined, using the given pH versus enzyme activity graph for enzyme A and B.
Concept Introduction:
Enzymes are defined as a biological catalyst for various reactions in the living organisms. Enzymes are like all catalyst as it also increases the rate reaction but they themselves do not changed permanently during the process. Enzyme does not alter the position of equilibrium and the relative energy of both the reactant and the products. Reaction when catalyzed by an enzyme increases the rate of reaction by
Answer to Problem 77P
Optimum pH for enzyme 'B' is
Explanation of Solution
The activity of enzyme is affected by both the temperature and pH. By increasing temperature, rate of reaction also increases until its maximum activity occurs that is at
Optimum pH is the pH at which the activity of an enzyme is maximum. So, from the given graph, the activity of enzyme B is maximum at pH 10.
Hence, the optimum pH of enzyme B is 10.
(c)
Interpretation:
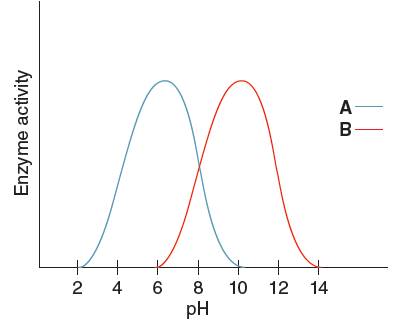
The enzyme having the higher activity when pH is
Concept Introduction:
Enzymes are defined as a biological catalyst for various reactions in the living organisms. Enzymes are like all catalyst as it also increases the rate reaction but they themselves do not changed permanently during the process. Enzyme does not alter the position of equilibrium and the relative energy of both the reactant and the products. Reaction when catalyzed by an enzyme increases the rate of reaction by
Answer to Problem 77P
Enzyme 'A' will have higher pH at
Explanation of Solution
The activity of enzyme is affected by both the temperature and pH. Optimum pH is the pH value at which the enzyme is most active. The rate of enzyme catalyzed reaction is maximum at optimum pH
The graph shown below clearly explains the activity of an enzyme with change in pH values:
From the given graph, it can be seen that the activity of enzyme B starts from pH 6 onwards and activity of enzyme A starts from pH 2 onwards. So, before pH 2 enzyme A is inactive and before pH 6 enzyme B is inactive. Therefore, at pH 4 the activity of enzyme A is higher than that of enzyme B.
(d)
Interpretation:
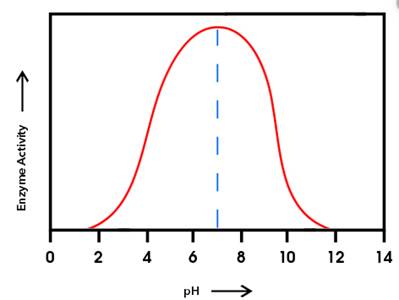
The enzyme having the higher activity at pH
Concept Introduction:
Enzymes are defined as a biological catalyst for various reactions in the living organisms. Enzymes are like all catalyst as it also increases the rate reaction but they themselves do not changed permanently during the process. Enzyme does not alter the position of equilibrium and the relative energy of both the reactant and the products. Reaction when catalyzed by an enzyme increases the rate of reaction by
Answer to Problem 77P
Enzyme 'B' will have the higher activity at pH
Explanation of Solution
The activity of enzyme is affected by both the temperature and pH. Optimum pH is the pH value at which the enzyme is most active. The rate of enzyme catalyzed reaction is maximum at optimum pH
The graph shown below clearly explains the activity of an enzyme with change in pH values:
From the given graph, it can be seen that the activity of enzyme B starts from pH 6 onwards and becomes maximum at pH 10and then activity of enzyme starts decreasing. And activity of enzyme A starts from pH 2 onwards and becomes maximum at pH 6 and then starts decreasing.
At pH 9, activity of enzyme A is decreasing and that of enzyme B is increasing. Hence, at pH 9 activity of enzyme B is higher than that of enzyme A.
Want to see more full solutions like this?
Chapter 21 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
- So, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forwardDraw a mental model for calcium chloride mixed with sodium phosphatearrow_forward
- here is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forwardDrawing of 3-fluro-2methylphenolarrow_forward
- Which compound(s) will be fully deprotonated (>99%) by reaction with one molar equivalent of sodium hydroxide? I, II, III I, || I, III I only II, III SH | H3C-C=C-H || III NH2arrow_forwardWill NBS (and heat or light) work for this reaction, or do we have to use Br2?arrow_forwardHAND DRAWarrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





