
Concept explainers
a. How many amide bonds join the amino acids in the peptide chain?
b.Give the name of the N-terminal amino acid.
c.Give the name of the C-terminal amino acid. 
(a)
Interpretation:
The number of amide bonds that join the amino acids in the: IRCFITPDIT-51 amino acid-CNPFPTRKRP peptide chain.
Concept Introduction:
Amino acids are organic compounds containing two functional groups namely amino and carboxylic group. The amino group is attached to the alpha carbon, carbon adjacent to the carbonyl group making them alpha-amino acids.
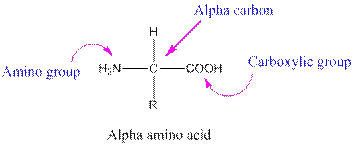
Answer to Problem 38P
There are
Explanation of Solution
When amino acids joined to one another by amide bonds to form large molecules are called peptides. The amide bonds present in peptides are called peptides bonds.
To make a dipeptide, the amino group
To calculate the number of amide bonds present in any sequence will be one less the number of amino acids present in the sequence.
For example, there are two amino acids present in a dipeptide. Then, the number of amide bonds will be one.
The given sequence is as follows:
IRCFITPDIT-51 amino acid-CNPFPTRKRP
There are total
(b)
Interpretation:
The name of the N-terminal amino acid of the given amino acid sequence present in the bee venom should be determined.
IRCFITPDIT-51 amino acid-CNPFPTRKRP
Concept Introduction:
Amino acids are organic compounds containing two functional groups namely amino and carboxylic group. The amino group is attached to the alpha carbon, carbon adjacent to the carbonyl group making them alpha-amino acids.
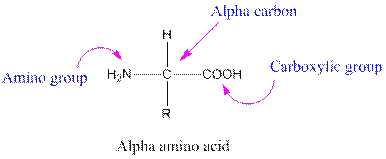
Answer to Problem 38P
Isoleucine (I) will be the N-terminal amino acid present in the given sequence IRCFITPDIT-51amino acid-CNPFPTRKRP.
Explanation of Solution
Amino acid having a free
The name, one-letter and three-letter abbreviation of the amino acids are mentioned in the table given below:
| Name of Amino Acid | Three-letter abbreviation | One-letter abbreviation |
| Alanine | Ala | A |
| Asparagine | Asn | N |
| Cysteine | Cys | C |
| Glutamine | Gln | Q |
| Glycine | Gly | G |
| Isoleucine | Ile | I |
| Leucine | Leu | L |
| Methionine | Met | M |
| Phenylalanine | Phe | F |
| Proline | Pro | P |
| Serine | Ser | S |
| Threonine | Thr | T |
| Tryptophan | Trp | W |
| Tyrosine | Tyr | Y |
| Valine | Val | V |
| Aspartic acid | Asp | D |
| Glutamic acid | Glu | E |
| Arginine | Arg | R |
| Histidine | His | H |
| Lysine | Lys | K |
The given sequence is as follows:
IRCFITPDIT-51 amino acid-CNPFPTRKRP
From the table, 'I' will be the amino acid present at the N-terminal. And 'I' is the 'Isoleucine' amino acid.
(c)
Interpretation:
The name of the C-terminal amino acid of the given amino acid sequence present in the bee venom should be determined.
IRCFITPDIT-51 amino acid-CNPFPTRKRP
Concept Introduction:
Amino acids are organic compounds containing two functional groups namely amino and carboxylic group. The amino group is attached to the alpha carbon, carbon adjacent to the carbonyl group making them alpha-amino acids.
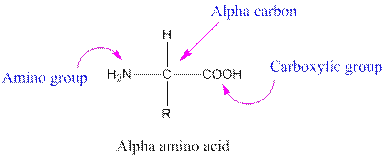
Answer to Problem 38P
Proline (P) will be the C-terminal amino acid present in the given sequence IRCFITPDIT-51amino acid-CNPFPTRKRP.
Explanation of Solution
Amino acid having a free
The name, one-letter and three-letter abbreviation of the amino acids are mentioned in the table given below:
| Name of Amino Acid | Three-letter abbreviation | One-letter abbreviation |
| Alanine | Ala | A |
| Asparagine | Asn | N |
| Cysteine | Cys | C |
| Glutamine | Gln | Q |
| Glycine | Gly | G |
| Isoleucine | Ile | I |
| Leucine | Leu | L |
| Methionine | Met | M |
| Phenylalanine | Phe | F |
| Proline | Pro | P |
| Serine | Ser | S |
| Threonine | Thr | T |
| Tryptophan | Trp | W |
| Tyrosine | Tyr | Y |
| Valine | Val | V |
| Aspartic acid | Asp | D |
| Glutamic acid | Glu | E |
| Arginine | Arg | R |
| Histidine | His | H |
| Lysine | Lys | K |
The given sequence is as follows:
IRCFITPDIT-51amino acid-CNPFPTRKRP
From the table, 'P' will be the amino acid present at the C-terminal. And 'P' is the 'Proline' amino acid.
Want to see more full solutions like this?
Chapter 21 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
- Choose the best reagents to complete the following reaction. i H A B 1. CH3CH2Na 2. H3O+ 1. CH3CH2MgBr 2. H3O+ 1. CH3MgBr Q C 2. H3O+ 1. H3O+ D 2. CH3MgBr 00 OH Q E CH³MgBrarrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. What is the half-life for this reaction?arrow_forwardChoose the best reagents to complete the following reaction. 1. PhNa A 2. H3O+ 1. PhCH2MgBr B 2. H3O+ хё 1. PhMgBr C 2. H3O+ 00 HO Q E D 1. H3O+ 2. PhMgBr PhMgBrarrow_forward
- Please answer all of the questions and provide detailed explanations and include a drawing to show the different signals on the molecule and include which ones should be highlighted.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 1 attempt remaining 1. LiAlH4 2. H3O+ Q OH ☑ Select to Drawarrow_forwardHow should I graph my data for the Absorbance of Pb and Fe for each mushroom? I want to compare the results to the known standard curve. Software: Excel Spreadsheets Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/Eb2PfHdfEtBJiWh0ipHZ_kkBW4idWWwvpLPPtqoq2WkgbQ?rtime=HxrF0_tR3Ugarrow_forward
- Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly, but do not use italics in Canvas.arrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 Marrow_forwardWhat is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)arrow_forward
- For reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardThe reaction of 2-oxacyclopentanone with hydrochloric acid in water (i.e., "excess") produces which of the following carboxylic acids?arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning





