
Concept explainers
Interpretation:
Lewis structure of
Concept introduction:
The structure of ammonia is,
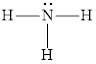
The nitrogen-containing organic compounds are known as amines. They are basic in nature and lone pairs of electrons are present on the nitrogen atom. They can be called as derivatives of ammonia. Alkyl group substitution for the hydrogen atoms in the ammonia molecule forms an amine.
Amines are named by the alkyl groups first in the order of alphabetical order followed by the suffix amine. When two or more than two same alkyl groups are present, then they are named as di-, tri-, tetra- and so on.
Lewis diagram is the representation of the molecule or ion. It shows the bonding between the atoms and also shows the unshared pairs of electrons on an atom.
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- Using Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forwardBA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a) NHBoc ⚫OBn HO. H3C CO2CH3 -OBn H3C H3C. H3C. NHBOC CI CO2CH3arrow_forward
- Draw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forwardUsing Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode. DATA: Electrode potentials E° = 0,854 V y E 0,788 V Hg2+/Hg 2+ Hg2/Hgarrow_forward1) NaNH2 (excess) 1) NaNH2 CI CI 2) H₂O 2) Mel 1) 03 2) (CH3)2S Na NH3 (liquid) 1arrow_forward
- CI 1) n-BuLi 2) 1) 03 HH T&Cl 2) H₂O 2arrow_forwardHelp with a!arrow_forwardFor the following compound: HO -H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: H-O: H-OH H-O HH H-OO Mechanism B: H-Q Mechanism C: Θ OH H-O: Mechanism D: H-O H- H-OO C H-OO H- H- H-OO HH OH -H - HON H :OH H-Harrow_forward
- identify the product (or multiple products) for each of the following reactions: CI 1) NaNH2 (excess) ठ Cl 2) H₂O Hz H₂SO₂, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) 1)9-BBN 2) H₂O, NaOH ? Br H A B C afó gó H OA B O c OD E OF D E F H H Na, NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: ? or CI CI 1) NaNHz (excess) 2) H₂O OA OB O C OD OE OF H₂SO₂, H₂O Hq50. 1) n-BuLi 2) Br 1) 9-BBN 2) H₂O₂, NaOH A B H H متته D E H H H H C H H F H H H₂ Lindlar's catalyst Na NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: O A OB Oc OD OE OF CI CI 1) NaNH2 (excess) 2) H₂O H₂ H₂SO2, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) Br 1)9-BBN 2) H₂O₂, NaOH ? Na, NH3 C H A H H مننه مننه منن مننه H F H H E مند H D H Harrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





