
Concept explainers
(a)
Interpretation:
From the following hydrocarbons, alkane is to be identified.
Concept introduction:
Hydrocarbons contain carbon and hydrogen atoms.
Answer to Problem 21.3TC
Among the given hydrocarbons
Explanation of Solution
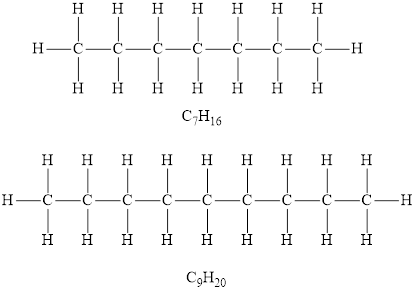
Alkanes are the saturated hydrocarbons in which each carbon atom is bonded to four other atoms via single bond only. The alkanes general formula of is
According to the general formula of alkanes, for 7 carbon atoms there must be 16 hydrogen atoms and for 9 carbon atoms, there must be 20 hydrogen atoms. Hydrocarbons
According to the general formula of alkanes, for 5 carbon atoms there must be 12 hydrogen atoms and for 11 carbon atoms, there must be 24 hydrogen atoms. Hydrocarbons
Among the given hydrocarbons
(b)
Interpretation:
The formula of the alkyl group derived from pentane is to be identified.
Concept introduction:
An alkyl group is derived from the respective alkane. Alkyl group is an alkane with one hydrogen atom less than the respective alkane. The alkyl group is named after alkane counterpart containing the same number of carbon atoms by replacing the “ane” suffix to “yl”.
Answer to Problem 21.3TC
The formula of the alkyl group obtained from pentane is
Explanation of Solution
The molecular formula of pentane is
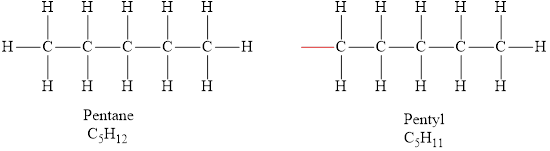
The formula of the alkyl group derived from pentane is
(c)
Interpretation:
The structural diagram of
Concept introduction:
Lewis diagram is the representation of the molecules or ions. It shows the bonding between the atoms and also shows the unshared pairs of electrons on an atom if any. The structural formula or structural diagram is nothing but Lewis structure without unshared pairs of electrons.
In the IUPAC name of an organic compound represents the number of the substituent and the numbers before the Greek prefix represent the position of the substituents. The suffix for an alkane is ‘ane. The number of carbon atoms in the parent hydrocarbon chain is represented by the root name. For example: ‘prop’ represent 3 carbon atoms present in the parent hydrocarbon chain.
Answer to Problem 21.3TC
The structural diagram of
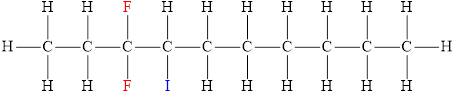
Explanation of Solution
The IUPAC name of the hydrocarbon is
The structura diagram is,
The structural diagram of

(d)
Interpretation:
The structural diagram of
Concept introduction:
Lewis diagram is the representation of the molecule or ion. It shows the bonding between the atoms and also shows the unshared pairs of electrons on an atom if any. The structural formula or structural diagram is nothing but Lewis structure without unshared pairs of electrons.
In the IUPAC name of an organic compound, the Greek prefix represents the number of the substituent and the numbers before the Greek prefix represent the position of the substituents. The suffix for an alkane is ‘ane and the prefix ‘cyclo’ is used for cycloalkanes. The number of carbon atoms in the parent hydrocarbon chain is represented by the root name. For example: ‘prop’ represent 3 carbon atoms present in the parent hydrocarbon chain.
Answer to Problem 21.3TC
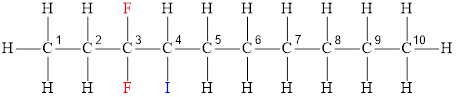
The structural diagram of
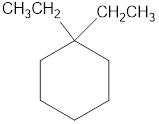
Explanation of Solution
The IUPAC name of the hydrocarbon is
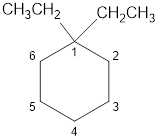
The structural diagram of

Want to see more full solutions like this?
Chapter 21 Solutions
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- How many particles does a face-centered cubic (fcc) unit cell contain? Group of answer choices 2 14 8 4arrow_forwardV Highlight all of the carbon atoms that have at least one beta (B) hydrogen, using red for one ẞ hydrogen, blue for two ẞ hydrogens, and green for three ẞ hydrogens. If none of the carbon atoms have ẞ hydrogens, check the box underneath the molecule. ED X None of the carbon atoms have ẞ hydrogens. Explanation esc 2 Check * F1 F2 1 2 80 # 3 Q W tab A caps lock shift fn control F3 N S option O 694 $ F4 F5 F6 005 % E R D F LL 6 olo 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility A DII F7 F8 87 & * 8 T Y U G H 4 F9 F10 ( 9 0 E F11 F12 உ J K L + || X C V B N M H H command option commandarrow_forwardConsider the reaction below and answer the following questions. Part 1 of 4 Br NaOCH2CH3 Identify the mechanisms involved. Check all that apply. SN 1 SN 2 E1 E2 None of the above Part 2 of 4 Skip Part Check esc F1 F2 lock 1 2 Q W A S #3 80 F3 F4 F5 F6 Save For © 2025 McGraw Hill LLC. All Rights Reserved. Terms ˇˇ % & 4 5 6 89 7 IK A 分 བ F7 F8 F9 F * E R T Y U 8 9 D F G H K V B N M 0 Oarrow_forward
- What kind of holes are not generated when solid-state particles adopt a close packing pattern? Group of answer choices tetrahedral cubic octahedral None of the other choices are correctarrow_forwardFor the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. 田 Major Product: Check ☐ + I Na OH esc F1 F2 2 1 @ 2 Q W tab A caps lock S #3 80 F3 69 4 σ F4 % 95 S Click and drag to sta drawing a structure mm Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use GO DII F5 F6 F7 F8 F9 F10 6 CO 89 & 7 LU E R T Y U 8* 9 0 D F G H J K L Z X C V B N M 36arrow_forwardProblem 7 of 10 Draw the major product of this reaction. Ignore inorganic byproducts. S' S 1. BuLi 2. ethylene oxide (C2H4O) Select to Draw a Submitarrow_forward
- Feedback (4/10) 30% Retry Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the reactant and missing intermediates involved in this reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Incorrect, 6 attempts remaining :0: Draw the Reactant H H3CO H- HIO: Ö-CH3 CH3OH2* protonation H. a H (+) H Ο CH3OH2 O: H3C protonation CH3OH deprotonation > CH3OH nucleophilic addition H. HO 0:0 Draw Intermediate a Xarrow_forwardCan I please get the blank spaces answered/answers?arrow_forward1. Identify the following alkenes as E or Z NH₂ Br 2. Draw the structures based on the IUPAC names (3R,4R)-3-bromo-4-fluoro- 1-hexene (Z)-4-bromo-2-iodo-3-ethyl- 3-heptene تر 3. For the following, predict all possible elimination product(s) and circle the major product. HO H₂SO4 Heat 80 F4 OH H2SO4 Heat 어요 F5 F6 1 A DII 4 F7 F8 F9 % & 5 6 7 * ∞ 8 BAB 3 E R T Y U 9 F D G H J K O A F11 F10arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. ○ O 1. H₂O, pyridine 2. neutralizing work-up a N W X 人 Parrow_forward✓ Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. tab OH CI 0 Br xx Br None of these molecules have a total of five ẞ hydrogens. esc Explanation Check caps lock shift 1 fn control 02 F2 W Q A N #3 S 80 F3 E $ t 01 205 % 5 F5 & 7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility FT * 8 R T Y U כ F6 9 FIG F11 F D G H J K L C X V B < N M H option command P H + F12 commandarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts and the carboxylic acid side product. O 1. CHзMgBr (excess) 2. H₂O ✓ W X 人arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





