
Concept explainers
Draw all the structural isomers for C8H18 that have the following root name (longest carbon chain). Name the structural isomers.
a. hexane
b. pentane
(a)
Interpretation: The structural isomers of
Concept introduction: Rules given by IUPAC should be followed to name an organic compound. Any organic compound has only one name that denotes that compound. The root word determines the number of carbons while counting the longest carbon chain. If more than one substituent is present, prefixes like di, tri, tetra, etc. are used and different substituents are written in alphabetical order.
Answer to Problem 16E
Answer
The structural isomers of
Explanation of Solution
Explanation
To determine: The structural isomers of
The structural isomer is given below and its name is
The structure of the isomer is,
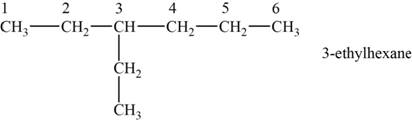
Figure 1
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Ethyl group is attached to third carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
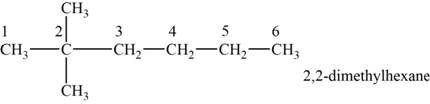
Figure 2
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Two methyl groups are attached to second carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the given isomer is,
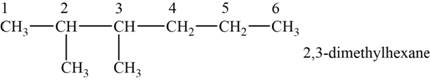
Figure 3
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl group is attached to second and third carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
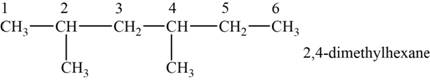
Figure 4
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl group is attached to second and fourth carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
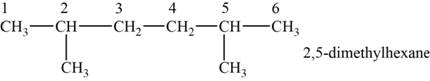
Figure 5
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl group is attached to second and fifth carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
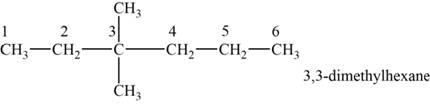
Figure 6
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Two Methyl groups are attached to third carbon, thus the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
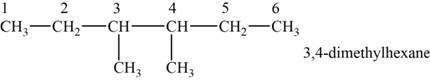
Figure 7
The general formula of alkanes is
Octane has eight carbons and
The isomer has six carbons in the parent chain. Therefore the root word “hexane” is used. Methyl groups are attached to third and fourth carbon, thus the name of the isomer is
Conclusion
The structural isomers of
(b)
Interpretation: The structural isomers of
Concept introduction: Rules given by IUPAC should be followed to name an organic compound. Any organic compound has only one name that denotes that compound. The root word determines the number of carbons while counting the longest carbon chain. If more than one substituent is present, prefixes like di, tri, tetra, etc. are used and different substituents are written in alphabetical order.
Explanation of Solution
Explanation
To determine: The structural isomers of
The structural isomer is given below and its name is
The structure of the isomer is,
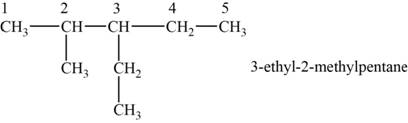
Figure 8
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Methyl group is attached to second carbon, ethyl group is attached to third carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
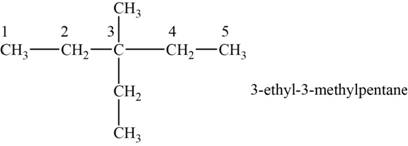
Figure 9
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Methyl group and ethyl group is attached to third carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
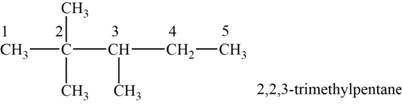
Figure 10
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Two methyl groups are attached to second carbon and one methyl group is attached to third carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
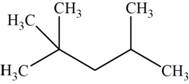
Figure 11
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Two methyl groups are attached to second carbon and one methyl group is attached to fourth carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
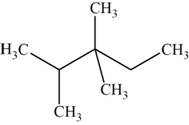
Figure 12
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Two methyl groups are attached to third carbon and one methyl group is attached to second carbon, therefore, the name of the isomer is
The structural isomer is given below and its name is
The structure of the isomer is,
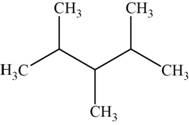
Figure 13
The general formula of alkanes is
Octane has eight carbons and
The isomer has five carbons in the parent chain. Therefore the root word “pentane” is used. Methyl group is attached to second, third carbon and fourth carbon, therefore, the name of the isomer is
Conclusion
The structural isomers of
Want to see more full solutions like this?
Chapter 21 Solutions
EBK CHEMISTRY: AN ATOMS FIRST APPROACH
- Chloroform, long used as an anesthetic and now considered carcinogenic, has a heat of vaporization of 31.4 kJ/mol. During vaporization, its entropy increases by 94.2 J/mol.K. Therefore, select the alternative that indicates the temperature, in degrees Celsius, at which chloroform begins to boil under a pressure of 1 atm. A) 28 B) 40 C) 52 D) 60 E) 72arrow_forwardIf we assume a system with an anodic overpotential, the variation of n as a function of current density: 1. at low fields is linear 2. at higher fields, it follows Tafel's law Obtain the range of current densities for which the overpotential has the same value when calculated for 1 and 2 cases (maximum relative difference of 5% compared to the behavior for higher fields). To which overpotential range does this correspond? Data: i = 1.5 mA cm², T = 300°C, B = 0.64, R = 8.314 J K1 mol-1 and F = 96485 C mol-1.arrow_forwardAnswer by equation pleasearrow_forward
- Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.arrow_forwardWhen talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forward
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





