
Concept explainers
(a)
Interpretation: The percent by mass of M in
Concept introduction: Grignard reagent is an
To determine: The percent by mass of M in
(a)
Answer to Problem 159IP
The mass percent of M in
Explanation of Solution
Given
Electronic configuration of
So,
In periodic table atomic number is found to be of the element Zinc.
The molar mass of Zinc is
The molar mass of compound
The molar mass of carbon is
The molar mass of hydrogen is
The molar mass of Zinc is
The molar mass of Bromine is
The molar mass of compound
Therefore the total molar mass of compound
The mass percent of M in
Substitute the value of the molar mass of M and total molar mass of compound, to calculate percent by mass of M in
Therefore, the mass percent of M in
The mass percent of M in
(b)
Interpretation: The percent by mass of M in
Concept introduction: Grignard reagent is an organometallic compound which is prepared by treating magnesium or metal atom with a strong acid. The Grignard reaction is defined as an organometallic reaction in which the vinyl, alkyl and aryl-metal halides were added to a carbonyl carbon atom and form an aldehyde or ketone.
To determine: The hybridization of the starred carbon atom in the given figure changed from reactants to products.
(b)
Answer to Problem 159IP
The hybridization of the starred carbon atom shown in Figure 1 is changed from
Explanation of Solution
The given reaction is,
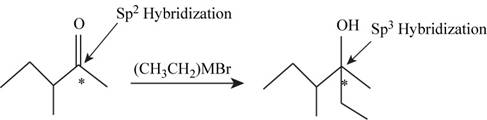
Figure 1
In the given reaction the reactant contains carbonyl group having double bonded oxygen atom hence, the hybridization of the starred carbon in the reactant is
The hybridization of the starred carbon atom shown in Figure 1 is changed from
(c)
Interpretation: The percent by mass of M in
Concept introduction: Grignard reagent is an organometallic compound which is prepared by treating magnesium or metal atom with a strong acid. The Grignard reaction is defined as an organometallic reaction in which the vinyl, alkyl and aryl-metal halides were added to a carbonyl carbon atom and form an aldehyde or ketone.
To determine: The systematic name of the product.
(c)
Answer to Problem 159IP
The systematic name of the product is
Explanation of Solution
The given compound is,
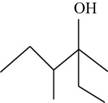
Figure 2
The longest carbon chain contains six carbon atoms numbered from the nearest
The systematic name of the product shown in Figure 2 is
Want to see more full solutions like this?
Chapter 21 Solutions
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
- pressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward
- 6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




