
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 20, Problem 48A
Interpretation Introduction
Interpretation:
The two commercially important alcohols are to be discussed.
Concept introduction:
Alcohol is a class of organic compound containing −OH as
Expert Solution & Answer
Answer to Problem 48A
Iso-propyl alcohol:
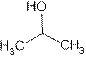
Ethylene glycol:
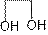
Explanation of Solution
Structure of Iso-propyl alcohol is:
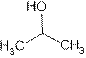
Iso-propyl alcohol is also known as propan-2-ol. Its uses are:
- As a solvent
- As a rubbing alcohol for skin
Structure of ethylene glycol is:
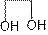
It is disubstituted alcohol. It is also known as ethane-1,2-
- As antifreeze
- As an ingredient in paints
- Reagent for many
organic synthesis
Conclusion
Thus, alcohol has application in many areas despite its drinking purpose.
Chapter 20 Solutions
World of Chemistry, 3rd edition
Ch. 20.1 - Prob. 1RQCh. 20.1 - Prob. 2RQCh. 20.1 - Prob. 3RQCh. 20.1 - Prob. 4RQCh. 20.1 - Prob. 5RQCh. 20.1 - Prob. 6RQCh. 20.1 - Prob. 7RQCh. 20.1 - Prob. 8RQCh. 20.2 - Prob. 1RQCh. 20.2 - Prob. 2RQ
Ch. 20.2 - Prob. 3RQCh. 20.2 - Prob. 4RQCh. 20.2 - Prob. 5RQCh. 20.2 - Prob. 6RQCh. 20.3 - Prob. 1RQCh. 20.3 - Prob. 2RQCh. 20.3 - Prob. 3RQCh. 20.3 - Prob. 4RQCh. 20.3 - Prob. 5RQCh. 20.4 - Prob. 1RQCh. 20.4 - Prob. 2RQCh. 20.4 - Prob. 3RQCh. 20.4 - Prob. 4RQCh. 20.4 - Prob. 5RQCh. 20 - Prob. 1ACh. 20 - Prob. 2ACh. 20 - Prob. 3ACh. 20 - Prob. 4ACh. 20 - Prob. 5ACh. 20 - Prob. 6ACh. 20 - Prob. 7ACh. 20 - Prob. 8ACh. 20 - Prob. 9ACh. 20 - Prob. 10ACh. 20 - Prob. 11ACh. 20 - Prob. 12ACh. 20 - Prob. 13ACh. 20 - Prob. 14ACh. 20 - Prob. 15ACh. 20 - Prob. 16ACh. 20 - Prob. 17ACh. 20 - Prob. 18ACh. 20 - Prob. 19ACh. 20 - Prob. 20ACh. 20 - Prob. 21ACh. 20 - Prob. 22ACh. 20 - Prob. 23ACh. 20 - Prob. 24ACh. 20 - Prob. 25ACh. 20 - Prob. 26ACh. 20 - Prob. 27ACh. 20 - Prob. 28ACh. 20 - Prob. 29ACh. 20 - Prob. 30ACh. 20 - Prob. 31ACh. 20 - Prob. 32ACh. 20 - Prob. 33ACh. 20 - Prob. 34ACh. 20 - Prob. 35ACh. 20 - Prob. 36ACh. 20 - Prob. 37ACh. 20 - Prob. 38ACh. 20 - Prob. 39ACh. 20 - Prob. 40ACh. 20 - Prob. 41ACh. 20 - Prob. 42ACh. 20 - Prob. 43ACh. 20 - Prob. 44ACh. 20 - Prob. 45ACh. 20 - Prob. 46ACh. 20 - Prob. 47ACh. 20 - Prob. 48ACh. 20 - Prob. 49ACh. 20 - Prob. 50ACh. 20 - Prob. 51ACh. 20 - Prob. 52ACh. 20 - Prob. 53ACh. 20 - Prob. 54ACh. 20 - Prob. 55ACh. 20 - Prob. 56ACh. 20 - Prob. 57ACh. 20 - Prob. 58ACh. 20 - Prob. 59ACh. 20 - Prob. 60ACh. 20 - Prob. 61ACh. 20 - Prob. 62ACh. 20 - Prob. 63ACh. 20 - Prob. 64ACh. 20 - Prob. 65ACh. 20 - Prob. 66ACh. 20 - Prob. 67ACh. 20 - Prob. 68ACh. 20 - Prob. 69ACh. 20 - Prob. 70ACh. 20 - Prob. 71ACh. 20 - Prob. 72ACh. 20 - Prob. 73ACh. 20 - Prob. 1STPCh. 20 - Prob. 2STPCh. 20 - Prob. 3STPCh. 20 - Prob. 4STPCh. 20 - Prob. 5STPCh. 20 - Prob. 6STPCh. 20 - Prob. 7STPCh. 20 - Prob. 8STPCh. 20 - Prob. 9STP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 24. What is the major product for the following reaction? Mg J. H.C CH H,C- Then H₂O OH Br C HO E HO H.C CH H.C- CH₂ CH₂ All of these are possiblearrow_forwardstructures. Explain why the major product(s) are formed over the minor product(s) using the Draw the major and product and the complete mechanism for all products with all resonance mechanism/resonance structures of the major and minor products in your explanation. HONO2 H2SO4arrow_forward#1 (a). Provide the expected product for the following reaction of A to B by indicating what the product is after step 1 (call this "81") and after step 2 (call this product "B2"). Give a complete mechanism for the transformation of compound A into compound B showing all intermediates, resonance structures, stereochemistry and electron movements 1. Et-MgBr 2. Me-Br B #1 (b). Compound A can be prepared in one step from an alkene starting material. Provide the structure a and the reaction conditions required to convert it to compound A The starting alkenearrow_forward
- The line formula for a branched alkene is shown below. 2 i. What is the molecular formula of this compound? Count number of C and H ii. How many carbon atoms are in the longest chain, ignoring the double bond? iii. What is the longest chain incorporating both carbons of the double bond? iv. How many substituents are on this chain? v. Give the IUPAC name for this compoundarrow_forwardgive the products for each of the followingarrow_forwardProvide the products and/or reagents for the following transformations. NaOMe HCl/EtOH OH NaOMe CI Show the product for the formation of the ketal given below for the transformation, showing all intermediates and resonance structures would be required to transform the ketal back to the starting ketone and then the mechanism What reagents/conditions HCI EtOH (excess)arrow_forward
- Make meta-dibromobenze from nitrobenzene using amine reactions. *see imagearrow_forwardProvide the structure of the expected major and minor (if any) products for each reaction. Clearly indicate stereochemistry where warranted. + + heat heat 이요 HNO3 1. AlCl3 2. H₂O H2SO4 1. AlCl3arrow_forward) Give the mechanism for the acid catalyzed hydrolysis of the following to the corresponding carboxylic acid. Show all intermediates and resonance structures N H+, H2O (excess)arrow_forward
- # 2. Drow full structures of the organic product expected in each of the following reactions. Draw the appropriate stereoisomer where warranted! Tos Cl O C NaCN PCC శ్రీ CI TSCI Pyridine H₂CrO4 PBrj Pyridine NaCNarrow_forwardPLEASE help. Locate a literature IR spectrum of eugenol. Insert the literature spectrum here: What conclusions can you draw about your clove oil from these IR spectra? I attached my data belowarrow_forwardplease help and the percent recovery of clove oil from cloves is 4.61% and i have attached my ir spectrum as well. Based on your GC data, how many components are in the clove oil? Calculate the percentage of each component. Clearly show your work. Which of the components corresponds to eugenol? How do you know? Is eugenol the major component?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY