
a.
Interpretation:The compounds C6H12 and C6H6 are to be named and their structures are to be drawn.
Concept Introduction:The carbon compounds are named according to IUPAC convention.
a.
Answer to Problem 5RQ
The compounds C6H12 and C6H6are called cyclohexane and benzene respectively.
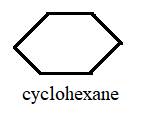
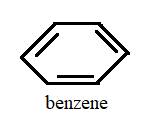
Explanation of Solution
The above structures represent C6H12 and C6H6 in stick form and they are standard compounds whose names are fixed by the IUPAC convention.
b.
Interpretation:The similarity of the compounds C6H12 and C6H6is to be written.
Concept Introduction:The similarity of the given compounds is primarily their structure.
b.
Answer to Problem 5RQ
The similarity of C6H12 and C6H6is that they are both cyclic compounds with a six carbon primary chain.
Explanation of Solution
Both cyclohexane and benzene have six carbon cyclic chain and both of them are planar so their bond angles and ring structure are also similar.
c.
Interpretation:Thedifferencebetween the compounds C6H12 and C6H6 is to be written.
Concept Introduction:Thedifference of the given compounds lies in their chemical properties.
c.
Answer to Problem 5RQ
C6H12is a saturated cycloalkane and is an aliphatic compound so it gives non sooty flame and has non-pleasant smell. On the other hand, C6H6is an unsaturated compound and is
Explanation of Solution
All the differences between cyclohexane and benzene is due to the resonating pi electron system of benzene which gives the compound its aromatic properties and makes it different from cyclohexane.
d.
Interpretation: Whether C6H12 will react by addition or substitution is to be written.
Concept Introduction: The preference for addition or substitution reactions depends on the presence of pi bonds.
d.
Answer to Problem 5RQ
C6H12prefers to undergo substitution reaction and not addition reaction.
Explanation of Solution
C6H12 is a saturated cycloalkane and all of its carbon atoms are sp3 hybrid with absence of any pi-bonding, therefore it cannot undergo addition reaction and prefers substitution reaction.
Chapter 20 Solutions
World of Chemistry, 3rd edition
- If I have 30% H2O2, indicate how to prepare a 6% H2O2 solution.arrow_forward7) 8) FCI II -C-C-C=C-C || Br Br || -C=C-Br -CEC-C-C- 10) 11) F Br i OH مله 12) Br i 13) 14) 15) CH3CHFCHFC=CH C(OH)Br2CHF(CH2)4CH2CH3 CH3(CH2)3CH=CH(CH2)2CH3arrow_forwardName 1) 3-fluoro, 1-butene 2) 2-heptene 2,3-difluoro- 1-pentene 4) 6-iodo,4-methyl- 2-decyne 5) 4,4-dibromo- 1,2-butandiol Complete structural formula F -C=C-C-C- Line formula Condensed structural formula N F CH2=CHCHFCH3arrow_forward
- 1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
- Assign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forward
- Predict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





