
Concept explainers
Interpretation: The appropriate option that suits for the similarity of an ester to a
Concept introduction: Carboxylic acid consists of carboxyl group, which contains two
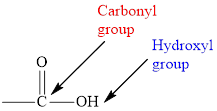
In carboxylic acid, the second oxygen atom is linked to a hydrogen atom.
The general formula for carboxylic acid is
Esters are carboxylic acid derivatives. The carbonyl group of the ester is polar. The general structure is,
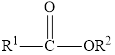
In an ester, the second oxygen atom is linked to another carbon atom.
The general formula for ester is
Answer to Problem 73A
An ester is similar to a carboxylic acid as a
Explanation of Solution
Carboxylic acid consists of carboxyl group, which contains two functional groups namely carbonyl and hydroxyl group.
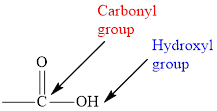
In carboxylic acid, the second oxygen atom is linked to a hydrogen atom.
The general formula for carboxylic acid is
Esters are carboxylic acid derivatives. The carbonyl group of the ester is polar and could take part in dipole-dipole attractions.
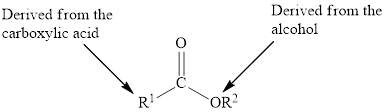
In an ester, the second oxygen atom is linked to another carbon atom.
The general formula for ester is
Ketones and aldehyde contain carbonyl group.
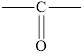
In ketones, the carbonyl group is bond to two carbon atoms. The general formula of a ketone is
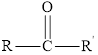
In a ketone, the carbonyl group is not present at the end of the hydrocarbon chain.
In aldehydes, the carbonyl group is attached at the end of the hydrocarbon chain. The carbonyl carbon is bonded to atleast one hydrogen.
Aldehydes have a general formula of
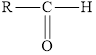
Here,
In an ester, an oxygen atom is attached to another carbon group bonded to the carbonyl carbon atom whereas carboxylic acid comprises of hydroxyl group attached to the carbonyl carbon atom.
In an aldehyde, one hydrogen atom is bonded to the carbonyl carbon atom whereas in ketone, two carbon groups are bonded to the carbonyl carbon atom.
An ester is similar to a carboxylic acid as a ketone is similar to an aldehyde. Option (e) is correct.
An ester is similar to a carboxylic acid is not like a primary alcohol similar to a secondary alcohol because in primary alcohol, the hydroxyl group is attached to single alkyl group whereas in secondary alcohol, the hydroxyl group is attached to two alkyl groups on either side.
The general formula of primary alcohol is
The general formula of secondary alcohol is
In an ester, an oxygen atom is attached to another carbon group bonded to the carbonyl carbon atom whereas carboxylic acid comprises of hydroxyl group attached to the carbonyl carbon atom.
The general formula for ester is
The general formula for carboxylic acid is
Here,
Option (a) is incorrect.
An ester is similar to a carboxylic acid is not like
The general formula of amine is
The general formula of ketone is
The general formula for ester is
The general formula for carboxylic acid is
Here,
Option (b) is incorrect.
An ester is similar to a carboxylic acid is not like ether similar to an aldehyde because ether (functional group containing oxygen atom bonded to two alkyl groups) does not a carbonyl carbon like aldehyde. Whereas an ester and carboxylic acid shows the presence of carbonyl carbon (in carboxylic acid, carbonyl carbon and hydroxyl group is collectively called as carboxyl group).
The general formula of ether is
The general formula of aldehyde is
The general formula for ester is
The general formula for carboxylic acid is
Here,
Option (c) is incorrect.
An ester is similar to a carboxylic acid is not like aromatic ring similar to a polymer because aromatic rings are hydrocarbons that contains benzene or some ring compounds. Polymers are repeated units of monomer, whereas an ester and carboxylic acid shows the presence of carbonyl carbon (in carboxylic acid, carbonyl carbon and hydroxyl group is collectively called as carboxyl group).
Option (d) is incorrect.
Chapter 20 Solutions
World of Chemistry, 3rd edition
- Write the systematic name of each organic molecule: structure 요 OH ہو۔ HO OH name X S ☐ ☐arrow_forwardPredict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. D ㄖˋ ید H No reaction. + 5 H₂O.* Click and drag to start drawing a structure. OH H₂Oarrow_forwardDraw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction 'O 10 + x 也 HO + 义 Click and drag to start drawing a structure.arrow_forward
- What are the angles a and b in the actual molecule of which this is a Lewis structure? H- :0: C=N: b Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = 0° b=0 Xarrow_forwardA student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. • If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + This transformation can't be done in one step. T iarrow_forwardDetermine the structures of the missing organic molecules in the following reaction: H+ O OH H+ + H₂O ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. X § ©arrow_forward
- Table 1.1 Stock Standard Solutions Preparation. The amounts shown should be dissolved in 100 mL. Millipore water. Calculate the corresponding anion concentrations based on the actual weights of the reagents. Anion Amount of reagent (g) Anion Concentration (mg/L) 0.1649 Reagent Chloride NaCl Fluoride NaF 0.2210 Bromide NaBr 0.1288 Nitrate NaNO3 0.1371 Nitrite NaNO2 0.1500 Phosphate KH2PO4 0.1433 Sulfate K2SO4 0.1814arrow_forwardDraw the structure of the pound in the provided CO as a 300-1200 37(2), 11 ( 110, and 2.5 (20arrow_forwardPlease help me with # 4 and 5. Thanks in advance!arrow_forward
- A small artisanal cheesemaker is testing the acidity of their milk before it coagulates. During fermentation, bacteria produce lactic acid (K₁ = 1.4 x 104), a weak acid that helps to curdle the milk and develop flavor. The cheesemaker has measured that the developing mixture contains lactic acid at an initial concentration of 0.025 M. Your task is to calculate the pH of this mixture and determine whether it meets the required acidity for proper cheese development. To achieve the best flavor, texture and reduce/control microbial growth, the pH range needs to be between pH 4.6 and 5.0. Assumptions: Lactic acid is a monoprotic acid H H :0:0: H-C-C H :0: O-H Figure 1: Lewis Structure for Lactic Acid For simplicity, you can use the generic formula HA to represent the acid You can assume lactic acid dissociation is in water as milk is mostly water. Temperature is 25°C 1. Write the K, expression for the dissociation of lactic acid in the space provided. Do not forget to include state symbols.…arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :0: :0 H. 0:0 :0: :6: S: :0: Select to Edit Arrows ::0 Select to Edit Arrows H :0: H :CI: Rotation Select to Edit Arrows H. < :0: :0: :0: S:arrow_forward3:48 PM Fri Apr 4 K Problem 4 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Mg. :0: Select to Add Arrows :0: :Br: Mg :0: :0: Select to Add Arrows Mg. Br: :0: 0:0- Br -190 H 0:0 Select to Add Arrows Select to Add Arrows neutralizing workup H CH3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





