
General, Organic, and Biological Chemistry
7th Edition
ISBN: 9781285853918
Author: H. Stephen Stoker
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.45EP
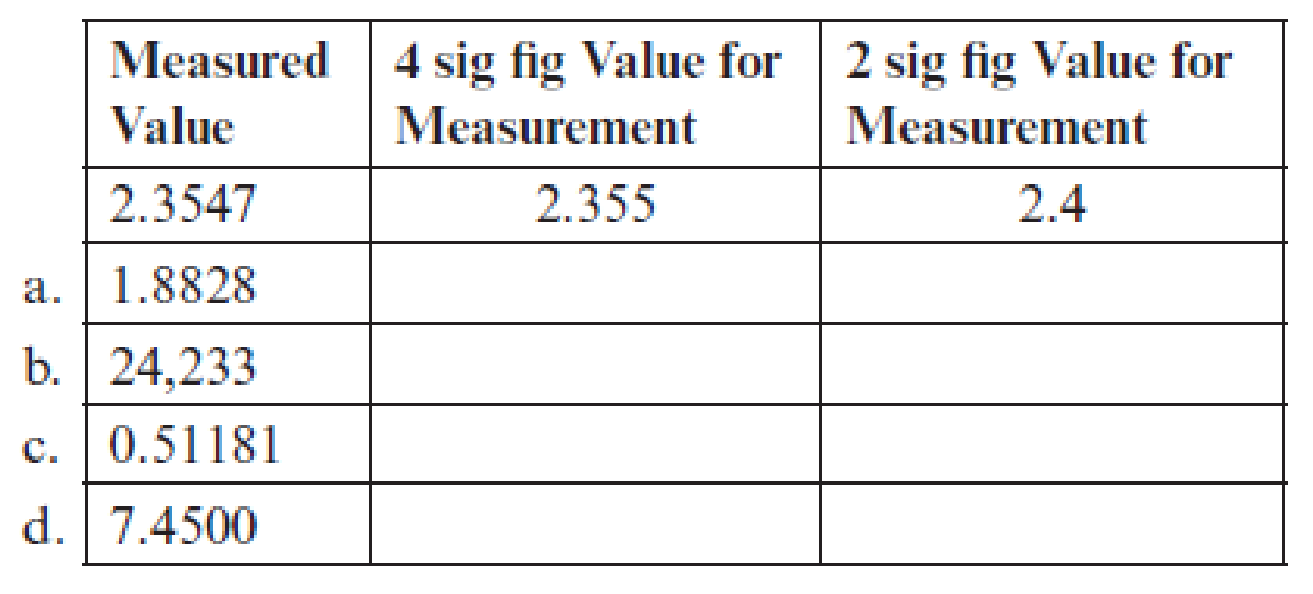
Complete the following table by filling in the blanks in each row with numerical values obtained by rounding the given values to the specified number of significant figures (sig figs). The first row is completed as an example.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the major products of this organic reaction.
2. Provide the structure of the major organic product in the following reaction. Pay particular
attention to the regio- and stereochemistry of your product.
H3CO
+
H
CN
A
Predict the major products of the following organic reaction.
Chapter 2 Solutions
General, Organic, and Biological Chemistry
Ch. 2.1 - Prob. 1QQCh. 2.1 - Preference by scientists for metric system unit...Ch. 2.2 - In which of the following pairings of metric...Ch. 2.2 - In which of the following sequences are the metric...Ch. 2.2 - Which of the following is a correct pairing of...Ch. 2.2 - Prob. 4QQCh. 2.2 - Prob. 5QQCh. 2.2 - Prob. 6QQCh. 2.2 - Prob. 7QQCh. 2.3 - Prob. 1QQ
Ch. 2.3 - Prob. 2QQCh. 2.4 - Prob. 1QQCh. 2.4 - Prob. 2QQCh. 2.4 - Prob. 3QQCh. 2.4 - Prob. 4QQCh. 2.4 - Prob. 5QQCh. 2.4 - Prob. 6QQCh. 2.5 - In which of the following cases is the given...Ch. 2.5 - When rounded to three significant figures, the...Ch. 2.5 - Prob. 3QQCh. 2.5 - Prob. 4QQCh. 2.6 - Prob. 1QQCh. 2.6 - Prob. 2QQCh. 2.6 - Prob. 3QQCh. 2.6 - Prob. 4QQCh. 2.6 - Prob. 5QQCh. 2.6 - Prob. 6QQCh. 2.7 - Prob. 1QQCh. 2.7 - Prob. 2QQCh. 2.7 - Which of the following is an incorrect conversion...Ch. 2.7 - Prob. 4QQCh. 2.8 - Prob. 1QQCh. 2.8 - Prob. 2QQCh. 2.9 - Prob. 1QQCh. 2.9 - Prob. 2QQCh. 2.9 - Prob. 3QQCh. 2.9 - What is the mass, in grams, of 30.0 mL of liquid...Ch. 2.10 - The freezing point of water is a. 0F b. 0 K c. 0C...Ch. 2.10 - Prob. 2QQCh. 2.10 - Prob. 3QQCh. 2.10 - Prob. 4QQCh. 2 - What is the main reason scientists prefer to use...Ch. 2 - List the more common types of measurements made in...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Arrange each of the following from smallest to...Ch. 2 - Arrange each of the following from smallest to...Ch. 2 - Which of the two given units is the more logical...Ch. 2 - Which of the two given units is the more logical...Ch. 2 - A person is told that there are 60 minutes in an...Ch. 2 - Prob. 2.12EPCh. 2 - Indicate whether the number in each of the...Ch. 2 - Indicate whether the number in each of the...Ch. 2 - Indicate whether each of the following quantities...Ch. 2 - Indicate whether each of the following quantities...Ch. 2 - Identify the estimated digit in each of the...Ch. 2 - Identify the estimated digit in each of the...Ch. 2 - Prob. 2.19EPCh. 2 - Prob. 2.20EPCh. 2 - Indicate to what decimal position readings should...Ch. 2 - Indicate to what decimal position readings should...Ch. 2 - Consider the following rulers as instruments for...Ch. 2 - Consider the following rulers as instruments for...Ch. 2 - Using the rulers given in Problem 2-23, what is...Ch. 2 - Using the rulers given in Problem 2-23, what is...Ch. 2 - With which of the rulers in Problem 2-23 was each...Ch. 2 - With which of the rulers in Problem 2-23 was each...Ch. 2 - Determine the number of significant figures in...Ch. 2 - Determine the number of significant figures in...Ch. 2 - Determine the number of significant figures in...Ch. 2 - Determine the number of significant figures in...Ch. 2 - In which of the following pairs of numbers do both...Ch. 2 - In which of the following pairs of numbers do both...Ch. 2 - Prob. 2.35EPCh. 2 - In the pairs of numbers of Problem 2-34, tell...Ch. 2 - Prob. 2.37EPCh. 2 - Complete the following table by filling in the...Ch. 2 - Prob. 2.39EPCh. 2 - The number of people present at an outdoor rock...Ch. 2 - Round off each of the following numbers to the...Ch. 2 - Round off each of the following numbers to the...Ch. 2 - Prob. 2.43EPCh. 2 - Round off (or add zeros) to each of the following...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Without actually solving, indicate the number of...Ch. 2 - Without actually solving, indicate the number of...Ch. 2 - Prob. 2.49EPCh. 2 - Carry out the following multiplications and...Ch. 2 - Carry out the following additions and...Ch. 2 - Carry out the following additions and...Ch. 2 - What is the uncertainty in the measured value...Ch. 2 - What is the uncertainty in the measured value...Ch. 2 - For each of the following numbers, will the...Ch. 2 - For each of the following numbers, will the...Ch. 2 - Prob. 2.57EPCh. 2 - Prob. 2.58EPCh. 2 - Prob. 2.59EPCh. 2 - For each of the numbers in Problem 2-56, how many...Ch. 2 - Express the following measured values in...Ch. 2 - Express the following measured values in...Ch. 2 - Change each of the following measured values from...Ch. 2 - Change each of the following measured values from...Ch. 2 - Prob. 2.65EPCh. 2 - Prob. 2.66EPCh. 2 - What is the uncertainty, in terms of a power of...Ch. 2 - What is the uncertainty, in terms of a power of...Ch. 2 - Write each of the following numbers in scientific...Ch. 2 - Write each of the following numbers in scientific...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Prob. 2.75EPCh. 2 - Indicate whether each of the following equations...Ch. 2 - Using dimensional analysis, convert each of the...Ch. 2 - Using dimensional analysis, convert each of the...Ch. 2 - The human stomach produces approximately 2500 mL...Ch. 2 - A typical loss of water through sweating for a...Ch. 2 - The mass of premature babies is customarily...Ch. 2 - The smallest bone in the human body, which is in...Ch. 2 - What volume of water, in gallons, would be...Ch. 2 - What volume of gasoline, in milliliters, would be...Ch. 2 - An individual weighs 83.2 kg and is 1.92 m tall....Ch. 2 - An individual weighs 135 lb and is 5 ft 4 in....Ch. 2 - Prob. 2.87EPCh. 2 - Prob. 2.88EPCh. 2 - Prob. 2.89EPCh. 2 - When each of the following measurements of mass is...Ch. 2 - A sample of mercury is found to have a mass of...Ch. 2 - A sample of sand is found to have a mass of 12.0 g...Ch. 2 - Acetone, the solvent in nail polish remover, has a...Ch. 2 - Silver metal has a density of 10.40 g/cm3. What is...Ch. 2 - The density of homogenized milk is 1.03 g/mL. How...Ch. 2 - Nickel metal has a density of 8.90 g/cm3. How much...Ch. 2 - Water has a density of 1.0 g/cm3 at room...Ch. 2 - Air has a density of 1.29 g/L at room temperature....Ch. 2 - Prob. 2.99EPCh. 2 - A two-gram sample of a red-colored liquid is found...Ch. 2 - Calculate the volume, in milliliters, for each of...Ch. 2 - Calculate the volume, in milliliters, for each of...Ch. 2 - An oven for baking pizza operates at approximately...Ch. 2 - A comfortable temperature for bathtub water is...Ch. 2 - Mercury freezes at 38.9C. What is the coldest...Ch. 2 - Prob. 2.106EPCh. 2 - Prob. 2.107EPCh. 2 - Which is the higher temperature, 15C or 4F?
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1) The isoamyl acetate report requires eight paragraphs - four for comparison of isoamyl alcohol and isoamyl acetate (one paragraph each devoted to MS, HNMR, CNMR and IR) and four for comparison of acetic acid and isoamyl acetate ((one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of…arrow_forwardWhat steps might you take to produce the following product from the given starting material? CI Br Он до NH2 NH2arrow_forward1) The isoamyl acetate report requires eight paragraphs - four for comparison of isoamyl alcohol and isoamyl acetate (one paragraph each devoted to MS, HNMR, CNMR and IR) and four for comparison of acetic acid and isoamyl acetate ((one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of…arrow_forward
- №3 Fill in the below boxes. HN 1. LAH 2. H3O+ NH2arrow_forwardFor the photochemical halogenation reaction below, draw both propagation steps and include the mechanism arrows for each step. H CH ot CH3 CI-CI MM hv of CH H-CI CH3 2nd attempt See Periodic Table See Hint Draw only radical electrons; do not add lone pair electrons. Note that arrows cannot meet in "space," and must end at either bonds or at atoms. 1 i Add the missing curved arrow notation to this propagation step. 20 H ن S F P H CI Br 品arrow_forwardThe radical below can be stabilized by resonance. 4th attempt Draw the resulting resonance structure. DOCEarrow_forward
- Use curved arrows to generate a second resonance form for the allylic radical formed from 2-methyl-2-pentene. 1 Draw the curved arrows that would generate a second resonance form for this radical. D 2 H S F A Бг Iarrow_forwardDraw the resulting product(s) from the coupling of the given radicals. Inlcude all applicable electrons and non-zero formal charges. H.C öö- CH3 2nd attempt +1 : 招 H₂C CH CH₂ See Periodic Table See H H C S F P Br CH₂ Iarrow_forwardPlease, help me out with the calculation, step by step on how to find what's blank with the given information.arrow_forward
- Predict the following products. Then show the mechanism. H₂N NH2arrow_forwardBF3, Boron Trifluoride, known to contain three covalent boron-fluorine bonds. suggest and illustrate all of the processes as well as their energetical consequences for the formation of BF3 from its elements.arrow_forwardDraw the mechanism of the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY