
General, Organic, and Biological Chemistry
7th Edition
ISBN: 9781285853918
Author: H. Stephen Stoker
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.23EP
Consider the following rulers as instruments for the measurement of length.
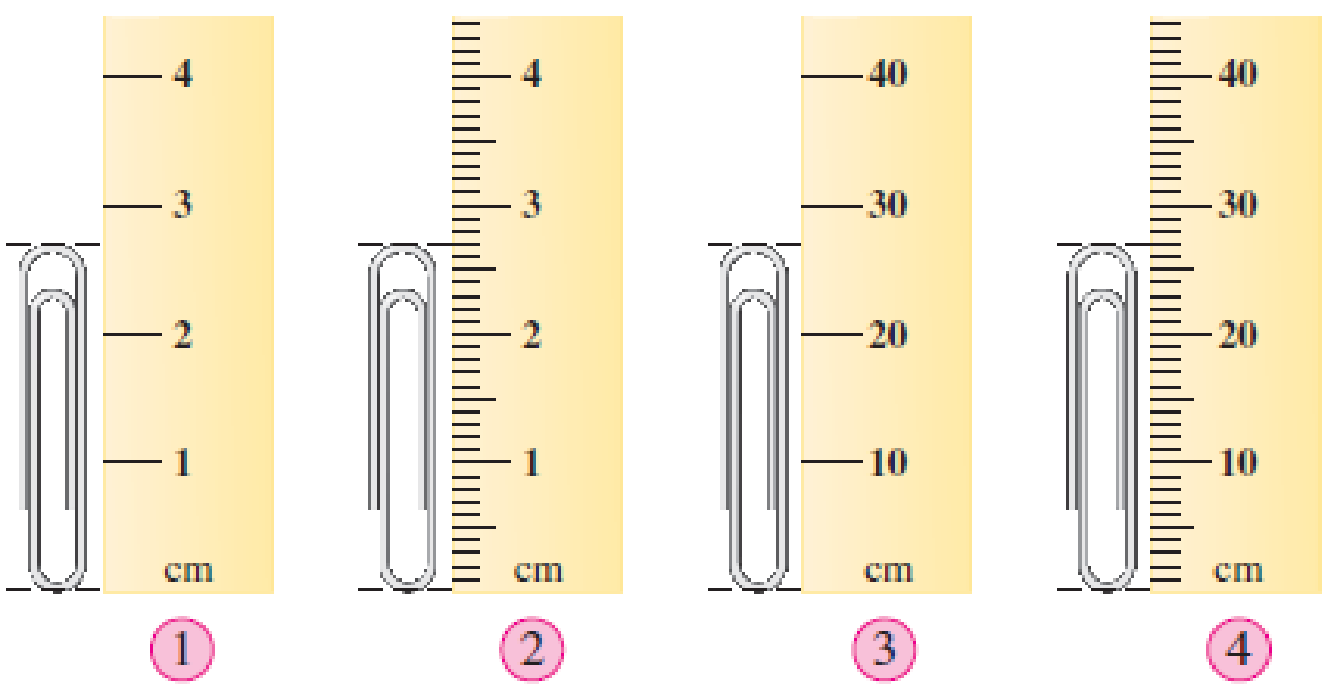
What would the uncertainty be in measurements made using the following?
- a. Ruler 1
- b. Ruler 4
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
9. OA. Rank the expected boiling points of the compounds shown below from highest to lowest. Place your answer
appropriately in the box. Only the answer in the box will be graded. (3) points)
OH
OH
بر بد بدید
2
3
There is an instrument in Johnson 334 that measures total-reflectance x-ray fluorescence (TXRF) to do elemental analysis (i.e., determine what elements are present in a sample). A researcher is preparing a to measure calcium content in a series of well water samples by TXRF with an internal standard of vanadium (atomic symbol: V). She has prepared a series of standard solutions to ensure a linear instrument response over the expected Ca concentration range of 40-80 ppm. The concentrations of Ca and V (ppm) and the instrument response (peak area, arbitrary units) are shown below. Also included is a sample spectrum. Equation 1 describes the response factor, K, relating the analyte signal (SA) and the standard signal (SIS) to their respective concentrations (CA and CIS).
Ca, ppm
V, ppm
SCa, arb. units
SV, arb. units
20.0
10.0
14375.11
14261.02
40.0
10.0
36182.15
17997.10
60.0
10.0
39275.74
12988.01
80.0
10.0
57530.75
14268.54
100.0…
A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C.
H₂O(g) + C₁₂O(g) = 2 HOCl(g)
K = 0.0900 at 25°C
с
Calculate the equilibrium concentrations of each gas at 25 °C.
[H₂O]=
[C₁₂O]=
[HOCI]=
M
Σ
M
Chapter 2 Solutions
General, Organic, and Biological Chemistry
Ch. 2.1 - Prob. 1QQCh. 2.1 - Preference by scientists for metric system unit...Ch. 2.2 - In which of the following pairings of metric...Ch. 2.2 - In which of the following sequences are the metric...Ch. 2.2 - Which of the following is a correct pairing of...Ch. 2.2 - Prob. 4QQCh. 2.2 - Prob. 5QQCh. 2.2 - Prob. 6QQCh. 2.2 - Prob. 7QQCh. 2.3 - Prob. 1QQ
Ch. 2.3 - Prob. 2QQCh. 2.4 - Prob. 1QQCh. 2.4 - Prob. 2QQCh. 2.4 - Prob. 3QQCh. 2.4 - Prob. 4QQCh. 2.4 - Prob. 5QQCh. 2.4 - Prob. 6QQCh. 2.5 - In which of the following cases is the given...Ch. 2.5 - When rounded to three significant figures, the...Ch. 2.5 - Prob. 3QQCh. 2.5 - Prob. 4QQCh. 2.6 - Prob. 1QQCh. 2.6 - Prob. 2QQCh. 2.6 - Prob. 3QQCh. 2.6 - Prob. 4QQCh. 2.6 - Prob. 5QQCh. 2.6 - Prob. 6QQCh. 2.7 - Prob. 1QQCh. 2.7 - Prob. 2QQCh. 2.7 - Which of the following is an incorrect conversion...Ch. 2.7 - Prob. 4QQCh. 2.8 - Prob. 1QQCh. 2.8 - Prob. 2QQCh. 2.9 - Prob. 1QQCh. 2.9 - Prob. 2QQCh. 2.9 - Prob. 3QQCh. 2.9 - What is the mass, in grams, of 30.0 mL of liquid...Ch. 2.10 - The freezing point of water is a. 0F b. 0 K c. 0C...Ch. 2.10 - Prob. 2QQCh. 2.10 - Prob. 3QQCh. 2.10 - Prob. 4QQCh. 2 - What is the main reason scientists prefer to use...Ch. 2 - List the more common types of measurements made in...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Arrange each of the following from smallest to...Ch. 2 - Arrange each of the following from smallest to...Ch. 2 - Which of the two given units is the more logical...Ch. 2 - Which of the two given units is the more logical...Ch. 2 - A person is told that there are 60 minutes in an...Ch. 2 - Prob. 2.12EPCh. 2 - Indicate whether the number in each of the...Ch. 2 - Indicate whether the number in each of the...Ch. 2 - Indicate whether each of the following quantities...Ch. 2 - Indicate whether each of the following quantities...Ch. 2 - Identify the estimated digit in each of the...Ch. 2 - Identify the estimated digit in each of the...Ch. 2 - Prob. 2.19EPCh. 2 - Prob. 2.20EPCh. 2 - Indicate to what decimal position readings should...Ch. 2 - Indicate to what decimal position readings should...Ch. 2 - Consider the following rulers as instruments for...Ch. 2 - Consider the following rulers as instruments for...Ch. 2 - Using the rulers given in Problem 2-23, what is...Ch. 2 - Using the rulers given in Problem 2-23, what is...Ch. 2 - With which of the rulers in Problem 2-23 was each...Ch. 2 - With which of the rulers in Problem 2-23 was each...Ch. 2 - Determine the number of significant figures in...Ch. 2 - Determine the number of significant figures in...Ch. 2 - Determine the number of significant figures in...Ch. 2 - Determine the number of significant figures in...Ch. 2 - In which of the following pairs of numbers do both...Ch. 2 - In which of the following pairs of numbers do both...Ch. 2 - Prob. 2.35EPCh. 2 - In the pairs of numbers of Problem 2-34, tell...Ch. 2 - Prob. 2.37EPCh. 2 - Complete the following table by filling in the...Ch. 2 - Prob. 2.39EPCh. 2 - The number of people present at an outdoor rock...Ch. 2 - Round off each of the following numbers to the...Ch. 2 - Round off each of the following numbers to the...Ch. 2 - Prob. 2.43EPCh. 2 - Round off (or add zeros) to each of the following...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Complete the following table by filling in the...Ch. 2 - Without actually solving, indicate the number of...Ch. 2 - Without actually solving, indicate the number of...Ch. 2 - Prob. 2.49EPCh. 2 - Carry out the following multiplications and...Ch. 2 - Carry out the following additions and...Ch. 2 - Carry out the following additions and...Ch. 2 - What is the uncertainty in the measured value...Ch. 2 - What is the uncertainty in the measured value...Ch. 2 - For each of the following numbers, will the...Ch. 2 - For each of the following numbers, will the...Ch. 2 - Prob. 2.57EPCh. 2 - Prob. 2.58EPCh. 2 - Prob. 2.59EPCh. 2 - For each of the numbers in Problem 2-56, how many...Ch. 2 - Express the following measured values in...Ch. 2 - Express the following measured values in...Ch. 2 - Change each of the following measured values from...Ch. 2 - Change each of the following measured values from...Ch. 2 - Prob. 2.65EPCh. 2 - Prob. 2.66EPCh. 2 - What is the uncertainty, in terms of a power of...Ch. 2 - What is the uncertainty, in terms of a power of...Ch. 2 - Write each of the following numbers in scientific...Ch. 2 - Write each of the following numbers in scientific...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Give the two forms of the conversion factor that...Ch. 2 - Prob. 2.75EPCh. 2 - Indicate whether each of the following equations...Ch. 2 - Using dimensional analysis, convert each of the...Ch. 2 - Using dimensional analysis, convert each of the...Ch. 2 - The human stomach produces approximately 2500 mL...Ch. 2 - A typical loss of water through sweating for a...Ch. 2 - The mass of premature babies is customarily...Ch. 2 - The smallest bone in the human body, which is in...Ch. 2 - What volume of water, in gallons, would be...Ch. 2 - What volume of gasoline, in milliliters, would be...Ch. 2 - An individual weighs 83.2 kg and is 1.92 m tall....Ch. 2 - An individual weighs 135 lb and is 5 ft 4 in....Ch. 2 - Prob. 2.87EPCh. 2 - Prob. 2.88EPCh. 2 - Prob. 2.89EPCh. 2 - When each of the following measurements of mass is...Ch. 2 - A sample of mercury is found to have a mass of...Ch. 2 - A sample of sand is found to have a mass of 12.0 g...Ch. 2 - Acetone, the solvent in nail polish remover, has a...Ch. 2 - Silver metal has a density of 10.40 g/cm3. What is...Ch. 2 - The density of homogenized milk is 1.03 g/mL. How...Ch. 2 - Nickel metal has a density of 8.90 g/cm3. How much...Ch. 2 - Water has a density of 1.0 g/cm3 at room...Ch. 2 - Air has a density of 1.29 g/L at room temperature....Ch. 2 - Prob. 2.99EPCh. 2 - A two-gram sample of a red-colored liquid is found...Ch. 2 - Calculate the volume, in milliliters, for each of...Ch. 2 - Calculate the volume, in milliliters, for each of...Ch. 2 - An oven for baking pizza operates at approximately...Ch. 2 - A comfortable temperature for bathtub water is...Ch. 2 - Mercury freezes at 38.9C. What is the coldest...Ch. 2 - Prob. 2.106EPCh. 2 - Prob. 2.107EPCh. 2 - Which is the higher temperature, 15C or 4F?
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?arrow_forwardProvide the structure, circle or draw, of the monomeric unit found in the biological polymeric materials given below. HO OH amylose OH OH 행 3 HO cellulose OH OH OH Ho HOarrow_forwardWhat units (if any) does K have? Does K depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)? in calculating the response factorarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardDon't used Ai solution and don't used hand raitingarrow_forwardOA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forward
- Don't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. CI Cl H3C-Cl CI a) A B C D Br Br b) A B C Br H3C-Br Darrow_forwardQ4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY