
Concept explainers
(a)
Interpretation:
Systematic name of the given compound has to be given.

Concept introduction:
IUAC gives rules for the naming of chemical compounds. These rules are,
For
In the nomenclature, Find the parent chin first then number the carbon atoms in the parent chain by giving lest number to the unsaturated double/triple bonded carbon followed by designate the substituent in its position in parent chain.
If more than one same substituent were occurs, add a prefix (di-, tri-, tetra-, ect..) in front of parent chain name.
If compound contains double bond means, name end with suffix of ene or if compound contains triple bond means name end with suffix of yne.
Finally the systematic name was written as, the substituent position number with prefix (di-, tri-, tetra-, ect..) of number of substituents followed by name of the substituent then write the parent chain name (which contains large number of carbon atoms in the chain) with suffix of suffix ene/yne.
The parent chain names based on the carbon atoms are,
The substituent names based on the carbon atoms are,
(b)
Interpretation:
Systematic name of the given compound has to be given.
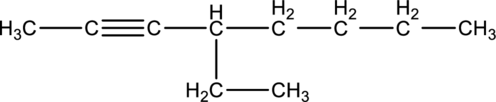
Concept introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- Draw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
- Provide the missing information. *see imagearrow_forwardI have a bottle of butanal that has been improperly used by lab workers. They allowed a traceamount NaOH (aq) to contaminate the bottle. What is now in my bottle of “butanal? What is the molecular name and functional group name? Draw the structure.arrow_forwardProvide the missing information. *see imagearrow_forward
- First image: Why can't the molecule C be formed in those conditions Second image: Synthesis for lactone C its not an examarrow_forwardFirst image: I have to show the mecanism for the reaction on the left, where the alcohol A is added fast in one portion Second image: I have to show the mecanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primaryarrow_forwardFirst image: I have to explain why the molecule C is never formed in those conditions. Second image: I have to propose a synthesis for the lactone Aarrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning


