
Concept explainers
(a)
Interpretation:
The systematic name and given alcohol should be given and it should be classified as primary, secondary or tertiary.
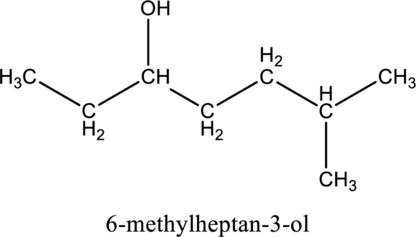
Concept introduction:
IUAC gives rules for the naming of chemical compounds. These rules are,
For saturated alcohols, longest chain with hydroxyl group is the parent chain of the compound and remaining groups and atoms are consider as substituent. In systematic name parent chain is ended with ol suffix.
In the nomenclature, Find the parent chin first then number the carbon atoms in the parent chain by giving lowest number to the hydroxyl substituted carbon followed by designate the other substituent in its position in parent chain.
If more than one same substituent or hydroxyl groups were occurs, add a prefix (di-, tri-, tetra-, ect..) in front of parent chain name.
Finally the systematic name was written as, the substituent position number with prefix (di-, tri-, tetra-, ect..) of number of substituents followed by name of the substituent then write the parent chain name (which contains large number of carbon atoms in the chain).
Alcohols:
In
In primary alcohols, hydroxyl group is connected with carbon, which is connected with all hydrogen or connected with single carbon substituent.
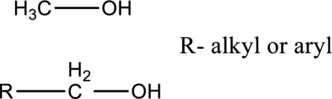
In secondary alcohols, the hydroxyl group is connected with carbon, which is connected two alkyl or aryl substituents.
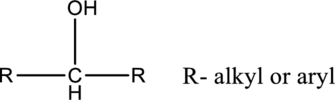
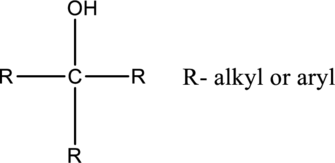
In tertiary alcohols, the hydroxyl group is connected with carbon, which is connected two alkyl or aryl substituents.
(b)
Interpretation:
The systematic name and given alcohol should be given and it should be classified as primary, secondary or tertiary.
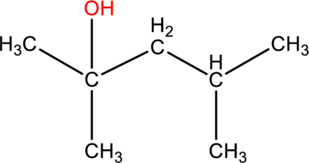
Concept introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





