
Concept explainers
Give the IUPAC name for each compound.
a. 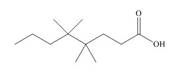 d.
d. 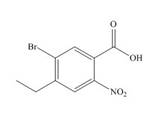
b. 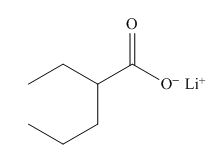 e.
e. 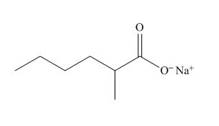
c. 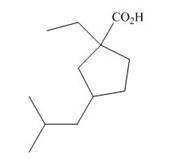 f.
f. 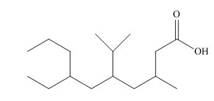
(a)
Interpretation: The IUPAC name for each compound is to be stated.
Concept introduction: International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of the parent name and the functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is the addition of substituents at appropriate carbon atoms.
Answer to Problem 19.29P
The name of the compound is
Explanation of Solution
The structure of the given compound is shown below.
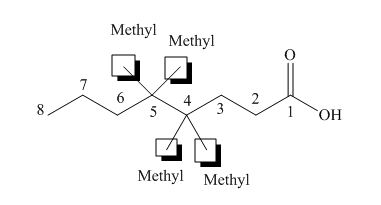
Figure 1
The above compound has a chain of eight carbon atoms. The carboxylic acid is present as a functional group in the compound. Two methyl groups are present at the fourth carbon atom and two methyl groups are present at the fifth carbon atom. Therefore, the name of the compound is
The name of the compound is
(b)
Interpretation: The IUPAC name for each compound is to be stated.
Concept introduction: International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of the parent name and the functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is the addition of substituents at appropriate carbon atoms.
Answer to Problem 19.29P
The name of the compound is lithium
Explanation of Solution
The structure of the given compound is shown below.
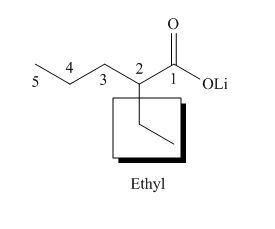
Figure 2
The above compound has a chain of five carbon atoms. The compound contains the salt of carboxylic acid as a functional group. Ethyl group is attached to the second carbon atom. Therefore, the name of the compound is lithium
The me of the compound is lithium
(c)
Interpretation: The IUPAC name for each compound is to be stated.
Concept introduction: International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of the parent name and the functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is the addition of substituents at appropriate carbon atoms.
Answer to Problem 19.29P
The name of the compound is
Explanation of Solution
The structure of the given compound is shown below.
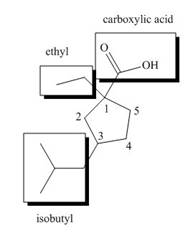
Figure 3
The above compound contains cyclopentyl ring. The carboxylic acid is present as a functional group in the compound. Ethyl and isobutyl group are attached to first and third carbon atoms respectively. Therefore, the name of the compound is
The name of the compound is
(d)
Interpretation: The IUPAC name for each compound is to be stated.
Concept introduction: International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of the parent name and the functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is the addition of substituents at appropriate carbon atoms.
Answer to Problem 19.29P
The name of the compound is
Explanation of Solution
The structure of the given compound is shown below.
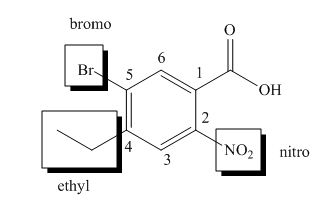
Figure 4
The above compound contains an aromatic ring. The carboxylic acid is present as a functional group in the compound. The nitro, ethyl and bromo groups are attached to second, fourth and fifth carbon atoms respectively. Therefore, the name of the compound is
The name of the compound is
(e)
Interpretation: The IUPAC name for each compound is to be stated.
Concept introduction: International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of the parent name and the functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is the addition of substituents at appropriate carbon atoms.
Answer to Problem 19.29P
The name of the compound is sodium
Explanation of Solution
The structure of the given compound is shown below.
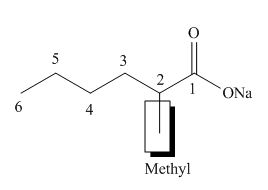
Figure 5
The above compound contains the chain of six carbon atoms. The salt of a carboxylic acid is a functional group in the compound. Methyl group is attached to second carbon atom. Therefore, the name of the compound is sodium
The name of the compound is sodium
(f)
Interpretation: The IUPAC name for each compound is to be stated.
Concept introduction: International Union of Pure and Applied Chemistry gave guidelines to write the chemical name of molecules. One should follow the given three steps to derive the structure of the compound from its IUPAC name. The first step involves the identification of the parent name and the functional group found at the end of the name. The second step is numbering of carbon skeleton in either direction. The third step is the addition of substituents at appropriate carbon atoms.
Answer to Problem 19.29P
The name of the compound is
Explanation of Solution
The structure of the given compound is shown below.
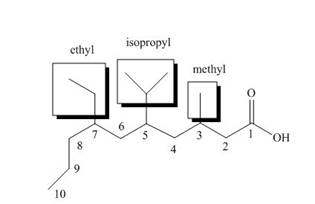
Figure 6
The above compound contains a chain of ten carbon atoms. The carboxylic acid is a functional group in the compound. The methyl, isopropyl and ethyl groups are attached to third, fifth and seventh carbon atom respectively. Therefore, the name of the compound is
The name of the compound is
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
- Provide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forwardGiven the attached data, provide the drawing for the corresponding structure.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


