
Concept explainers
(a)
Interpretation:The IUPAC name for the given compound is to be stated.
Concept introduction:The chemical structures are described by IUPAC name or common names. IUPAC nameis different from common names because IUPAC nameis applied at international level and it comprises suffix, prefix, numbers and other priority rules.
Answer to Problem 19.1P
The IUPAC name of the compound is
Explanation of Solution
The numbering of carbon atoms in the given compound is shown below.
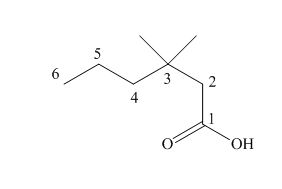
Figure 1
The given compound has a chain of six carbon atoms. The
The IUPAC name of the given compound is
(b)
Interpretation:The IUPAC name for the given compound is to be stated.
Concept introduction:The chemical structures are described by IUPAC name or common names. IUPAC name is different from common names because IUPAC name is applied at international level and it comprises suffix, prefix, numbers and other priority rules.
Answer to Problem 19.1P
The IUPAC name of the compound is
Explanation of Solution
The numbering of carbon atoms in the given compound is shown below.
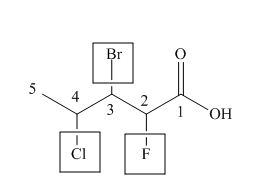
Figure 2
The given compound has a chain of five carbon atoms. The carboxylic acid is present as a functional group in the compound. Fluorine, chlorine and bromine is present at second, third and fourth carbon atoms respectively. Therefore, the name of the compound is
The IUPAC name of the compound is
(c)
Interpretation:The IUPAC name for the given compound is to be stated.
Concept introduction:The chemical structures are described by IUPAC name or common names. IUPAC name is different from common names because IUPAC name is applied at international level and it comprises suffix, prefix, numbers and other priority rules.
Answer to Problem 19.1P
The IUPAC name of the compound is
Explanation of Solution
The numbering of carbon atoms in the given compound is shown below.
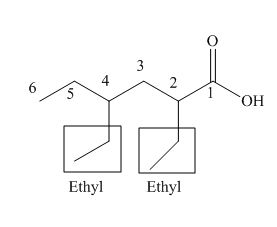
Figure 3
The given compound has a chain of six carbon atoms. The carboxylic acid is present as a functional group in the compound. Two ethyl groups are present at second and fourth carbon atoms. Therefore, the name of the compound is
The IUPAC name of the compound is
(d)
Interpretation:The IUPAC name for the given compound is to be stated.
Concept introduction:The chemical structures are described by IUPAC name or common names. IUPAC name is different from common names because IUPAC name is applied at international level and it comprises suffix, prefix, numbers and other priority rules.
Answer to Problem 19.1P
The IUPAC name of the compound is
Explanation of Solution
The numbering of carbon atoms in the given compound is shown below.
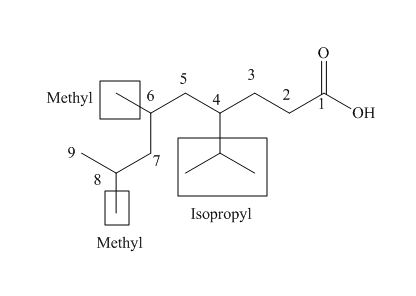
Figure 4
The given compound has a chain of nine carbon atoms. The carboxylic acid is present as a functional group in the compound. Two methyl groups are present at eighth and nine carbon atoms, while one isopropyl group is present at fourth carbon. Therefore, the name of the compound is
The IUPAC name of the compound is
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



