
Concept explainers
What alcohol can be oxidized to each
a. 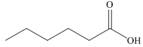 b.
b. 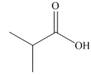 c.
c. 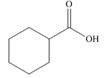
(a)
Interpretation: Alcohol that can be oxidized to the given carboxylic acid is to be identified.
Concept introduction: Carboxylic acids are the carbon compounds that contain carboxyl group as a major functional group. They are polar in nature due to electronegativity difference between the atoms in a compound. They sometimes exist as a dimer. Dimers are the compounds that consist of two monomer units connected by bonds or forces. Carboxylic acids can be synthesized from alkynes, alkene, benzene derivatives, alcohol and allylic halides by using different reagents.
Answer to Problem 19.10P
Alcohol that can be oxidized to the given carboxylic acid is
Explanation of Solution
The structure of alcohol that can be oxidized to the given carboxylic acid is shown below.
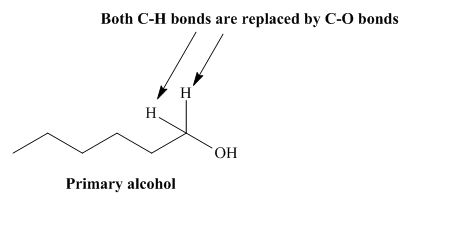
Figure 1
The compound in the figure is
Alcohol that can be oxidized to the given carboxylic acid is
(b)
Interpretation: Alcohol that can be oxidized to the given carboxylic acid is to be identified.
Concept introduction: Carboxylic acids are the carbon compounds that contain carboxyl group as a major functional group. They are polar in nature due to electronegativity difference between the atoms in a compound. These sometimes exist as a dimer. Dimers are the compounds that consist of two monomer units connected by bonds or forces. Carboxylic acids can be synthesized from alkynes, alkene, benzene derivatives, alcohol and allylic halides by using different reagents.
Answer to Problem 19.10P
Alcohol that can be oxidized to the given carboxylic acid is
Explanation of Solution
The given carboxylic acid is
Alcohol that can be oxidized to the given carboxylic acid is
(c)
Interpretation: Alcohol that can be oxidized to the given carboxylic acid is to be identified.
Concept introduction: Carboxylic acids are the carbon compounds that contain carboxyl group as a major functional group. They are polar in nature due to electronegativity difference between the atoms in a compound. These sometimes exist as a dimer. Dimers are the compounds that consist of two monomer units connected by bonds or forces. Carboxylic acids can be synthesized from alkynes, alkene, benzene derivatives, alcohol and allylic halides by using different reagents.
Answer to Problem 19.10P
Alcohol that can be oxidized to the given carboxylic acid is
Explanation of Solution
The structure of alcohol that can be oxidized to the given carboxylic acid is shown below.
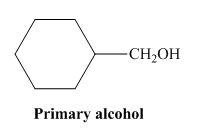
Figure 2
The compound in the figure is
Alcohol that can be oxidized to the given carboxylic acid is
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
- help draw the moleculearrow_forwardHow to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forward
- How can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





