
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 17PP
Practice Problem 19.17
What starting compound would you use in an aldol cyclization to prepare each of the following?
(a)
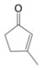
(b)
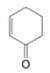
(c)
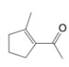
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a. Explain Why electron withdrawing groupe
tend to be meta-Directors. Your answer Should
lyclude all apropriate. Resonance contributing
Structures
6.
Explain why -ll is an ortho -pura
drccton evon though chlorine has a very High
Electronegativity
C.
Ν
H
a.
H3C.
N
H3C
CH3
HCN
Chapter 19 Solutions
Organic Chemistry
Ch. 19 - PRACTICE PROBLEM 19.1 (a) Write a mechanism. for...Ch. 19 - Practice Problem 19.2
Since the products obtained...Ch. 19 - Prob. 3PPCh. 19 - PRACTICE PROBLEM 19.4
Write mechanisms that...Ch. 19 - Prob. 5PPCh. 19 - Prob. 6PPCh. 19 - Practice Problem 19.7 The acid-catalyzed aldol...Ch. 19 - Prob. 8PPCh. 19 - Practice Problem 19.9
(a) Provide a mechanism for...Ch. 19 - Prob. 10PP
Ch. 19 - Practice Problem 19.11 Outlined below is a...Ch. 19 - Prob. 12PPCh. 19 - Prob. 13PPCh. 19 - Prob. 14PPCh. 19 - Practice Problem 19.15
Starting with ketones and...Ch. 19 - Practice Problem 19.16 Assuming that dehydration...Ch. 19 - Practice Problem 19.17 What starting compound...Ch. 19 - Practice Problem 19.18
What experimental...Ch. 19 - Prob. 19PPCh. 19 - Practice Problem 19.20
When acrolein (propenal)...Ch. 19 - Prob. 21PPCh. 19 - PRACTICE PROBLEM 19.22
Qutline reasonable...Ch. 19 - Prob. 23PCh. 19 - Show all steps in the following syntheses. You may...Ch. 19 - Prob. 25PCh. 19 - Prob. 26PCh. 19 - Prob. 27PCh. 19 - 19.28 Show how the diketone at the right could be...Ch. 19 - Prob. 29PCh. 19 - 19.30 Write a detailed mechanism for the following...Ch. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - 19.33 Predict the products from each of the...Ch. 19 - Prob. 34PCh. 19 - Show how each of the following transformations...Ch. 19 - Prob. 36PCh. 19 - What reagents would you use to bring about each...Ch. 19 - Prob. 38PCh. 19 - Prob. 39PCh. 19 - 19.40 When the aldol reaction of acetaldehyde is...Ch. 19 - Prob. 41PCh. 19 - Prob. 42PCh. 19 - 19.43 The following reaction illustrates the...Ch. 19 - What is the structure of the cyclic compound that...Ch. 19 - Prob. 45PCh. 19 - Prob. 46PCh. 19 - Prob. 47PCh. 19 - Predict the products from the following reactions....Ch. 19 - Prob. 49PCh. 19 - 19.50 (+)-Fenchone is a terpenoid that can be...Ch. 19 - Prob. 51PCh. 19 - Prob. 52PCh. 19 - Prob. 53PCh. 19 - Prob. 54PCh. 19 - The Perkin condensation is an aldol-type...Ch. 19 - 19.55 (a) Infrared spectroscopy provides an easy...Ch. 19 - Prob. 57PCh. 19 - 19.61 Provide a mechanism for each of the...Ch. 19 - 19.62 (a) Deduce the structure of product A,...Ch. 19 - 19.60 Write the structures of the three products...Ch. 19 - Prob. 1LGPCh. 19 - Which hydrogen atoms in the following ester are...Ch. 19 - Prob. 2QCh. 19 - 19.3 What starting materials could be used in a...Ch. 19 - Supply the missing reagents, intermediates, and...Ch. 19 - Prob. 5QCh. 19 - Prob. 6QCh. 19 - Prob. 7QCh. 19 - Prob. 8Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does the organism Prochlorococcus contribute to both the carbon and oxygen cycles in the oceans?
Brock Biology of Microorganisms (15th Edition)
The glycine cleavage system is a group of four enzymes that together catalyze the following reaction: glycine+T...
Organic Chemistry (8th Edition)
Compare the roles of CO2 and H2O in cellular respiration and photosynthesis.
Campbell Biology (11th Edition)
30. What structural and functional changes occur in the mother during pregnancy?
Principles of Anatomy and Physiology
30. Drosophila has a diploid chromosome number of 2n = 8, which includes one pair of sex chromosomes (XX in fem...
Genetic Analysis: An Integrated Approach (3rd Edition)
64. Determine the [H3O+] and pH of a 0.200 M solution of formic acid.
Chemistry: A Molecular Approach (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ол 2. восцапан (46:00) Curtius rearrangment 1. NaN3, heat -OHarrow_forwardQuestion 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forward
- Indicate what the Luther equation is used for?arrow_forwardIndicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forward
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forwardExplain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forwardExplain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forward
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License